761311
cataCXium® A Pd G2
Synonym(s):
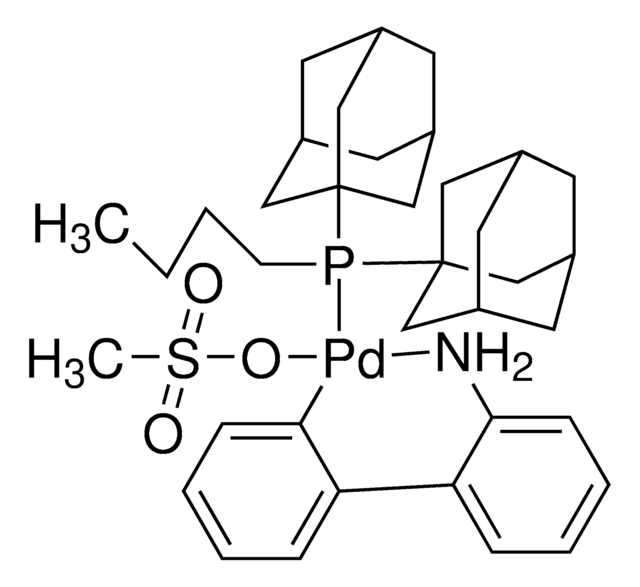
cataCXium A-Pd-G2, Chloro[(di(1-adamantyl)-N-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)
About This Item
Recommended Products
form
solid
Quality Level
feature
generation 2
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
220 °C (decomposition)
functional group
amine
phosphine
SMILES string
Nc1ccccc1-c2ccccc2[Pd]Cl.CCCCP([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C24H39P.C12H10N.ClH.Pd/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;;/h17-22H,2-16H2,1H3;1-6,8-9H,13H2;1H;/q;;;+1/p-1/t17-,18+,19-,20-,21+,22-,23-,24-;;;
InChI key
CAVPLNLGXHYGIR-DVBMAMJVSA-M
Application
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)





![5-[Di(1-adamantyl)phosphino]-1′,3′,5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/334/020/2da77724-fc86-4d88-bc93-0b43ae777daa/640/2da77724-fc86-4d88-bc93-0b43ae777daa.png)

![[1,1′-Bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)
