675903
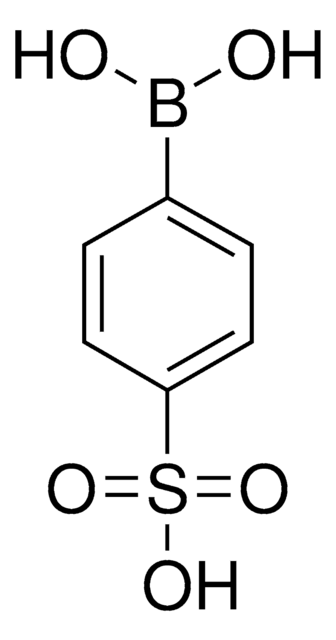
4-(Methanesulfonyl)phenylboronic acid
≥95.0%
Synonym(s):
4-(Methanesulfonyl)benzeneboronic acid, 4-(Methylsulfonyl)phenylboronic acid, 4-Methansulfonylphenylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(H3CSO2)C6H4B(OH)2
CAS Number:
Molecular Weight:
200.02
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
289-293 °C
functional group
sulfone
SMILES string
CS(=O)(=O)c1ccc(cc1)B(O)O
InChI
1S/C7H9BO4S/c1-13(11,12)7-4-2-6(3-5-7)8(9)10/h2-5,9-10H,1H3
InChI key
VDUKDQTYMWUSAC-UHFFFAOYSA-N
Related Categories
General description
Contains varying amounts of anhydride
Application
4-(Methanesulfonyl)phenylboronic acid may be used as reagent for:
Reagent used in Preparation of
- sequential Suzuki cross-coupling reactions
- Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids
- directed metalation and regioselective functionalization of 3-bromofuran and related heterocycles
- Barton-Zard pyrrole cyclocondensations and Baeyer-Villiger oxidations
- diplar cycloaddition and palladium-catalyzed cross-coupling processes
- continuous flow Suzuki reactions for odanacatib intermediate synthesis
Reagent used in Preparation of
- diarylaminopyridines as potential anti-malarial agents
- hydropyranopyrazine via chloropyrazinecarboxaldehyde and olefination
- biaryl sulfone derivatives as antagonists of the histamine H3 receptor
- novel kinase inhibitor scaffolds with potential antitumor effects
- Hepatitis C virus inhibition activity of N-hydroxyisoquinoline di
Highly effective boronic acid used in a rhodium-catalyzed asymmetric 1,4-addition to 4-oxobutenamides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids with TMSCF3 and elemental sulfur.
Chao Chen et al.
Angewandte Chemie (International ed. in English), 51(10), 2492-2495 (2012-01-31)
Synthesis of 2,6-disubstituted-7,8-dihydro-6H-pyrano[2,3-b]pyrazines
Li, J-C.; et al.
Tetrahedron Letters, 53, 852-853 (2012)
Jamie L Zigterman et al.
The Journal of organic chemistry, 72(23), 8870-8876 (2007-10-12)
A variety of 4-oxobutenamides 1 were subjected to rhodium-catalyzed conjugate addition with arylboronic acids providing high regio- and enantioselectivity (97:3 to >99:1, >96% ee) and moderate to excellent yields (54-99%). The key to high selectivity is the use of sterically
Optimization of a novel kinase inhibitor scaffold for the dual inhibition of JAK2 and FAK kinases
Zificsak, C. A.; et al.
Bioorganic & Medicinal Chemistry, 22, 133-137 (2012)
Pamela Kassis et al.
European journal of medicinal chemistry, 46(11), 5416-5434 (2011-09-29)
We here report the synthesis and biological evaluation of new 3-[(2-indolyl)]-5-phenyl-3,5-pyridine, 3-[(2-indolyl)]-5-phenyl-2,4-pyridine and 3-[(2-indolyl)]-5-phenyl-2,6-pyrazine derivatives designed as potential CDK inhibitors. Indoles and phenyls were used to generate several substitutions of the pyridine and pyrazine rings. The synthesis included Stille or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service