672033
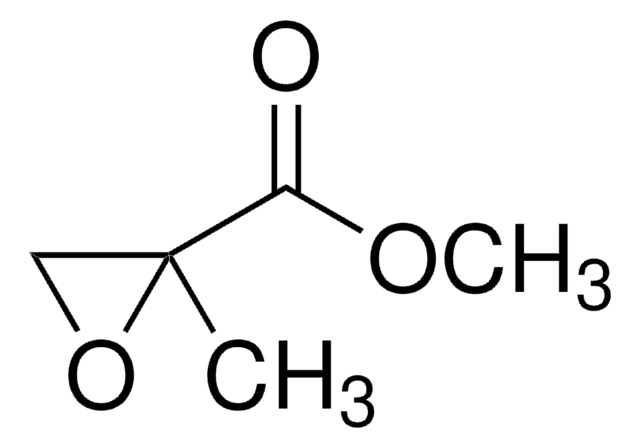
(S)-N-Boc-2,3-epoxypropylamine
97%
Synonym(s):
tert-Butyl (2S)-N-(2-oxiranylmethyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H15NO3
CAS Number:
Molecular Weight:
173.21
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥96.5% (GCF)
97%
form
solid
optical purity
ee: ≥99.0% (GC)
mp
48-50 °C
functional group
amine
ether
SMILES string
CC(C)(C)OC(=O)NC[C@H]1CO1
InChI
1S/C8H15NO3/c1-8(2,3)12-7(10)9-4-6-5-11-6/h6H,4-5H2,1-3H3,(H,9,10)/t6-/m0/s1
InChI key
ZBBGKXNNTNBRBH-LURJTMIESA-N
Related Categories
Application
(S)-N-Boc-2,3-epoxypropylamine can be used:
- To functionalize detonation nanodiamonds with NH2 functional group to enhance their optical properties.
- In the synthesis of poly(ethylene glycol) (PEG) based block copolymers, which are further used in the preparation of micelles loaded with mRNA for intracellular mRNA delivery.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guanidine-phosphate interactions stabilize polyion complex micelles based on flexible catiomers to improve mRNA delivery
Miyazaki T, et al.
European Polymer Journal, 140(1), 110028-110028 (2020)
Tandem malonate-based glucosides (TMGs) for membrane protein structural studies
Hussain H, et al.
Scientific reports, 7(1), 1-11 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service