667005
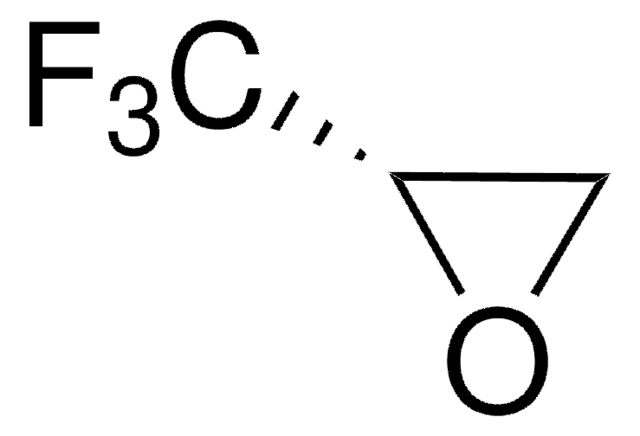
(R)-(+)-3,3,3-Trifluoro-1,2-epoxypropane
97%
Synonym(s):
(R)-(+)-2-(Trifluoromethyl)oxirane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H3F3O
CAS Number:
Molecular Weight:
112.05
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D <1.300
bp
25-32 °C
density
1.294 g/mL at 25 °C
functional group
ether
fluoro
storage temp.
2-8°C
SMILES string
FC(F)(F)[C@H]1CO1
InChI
1S/C3H3F3O/c4-3(5,6)2-1-7-2/h2H,1H2/t2-/m1/s1
InChI key
AQZRARFZZMGLHL-UWTATZPHSA-N
Related Categories
Application
(R)-(+)-3,3,3-Trifluoro-1,2-epoxypropane can be used as a substrate to synthesize:
- Substituted trifluoro amino propanols, which are found to be potent inhibitors of cholesteryl ester transfer protein.
- (2R) Trifluoro-(methoxybenzyloxy)-propanol (chiral glycol) by reacting with 4-methoxybenzyl alcohol in the presence of NaH. Chiral glycol intermediate is further utilized for the preparation of trifluoromethyl glycol carbamates as potential monoacylglycerol lipase (MAGL) inhibitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-14.8 °F
Flash Point(C)
-26 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation
McAllister LA, et al.
Journal of Medicinal Chemistry, 61(7), 3008-3026 (2018)
Discovery of a simple picomolar inhibitor of cholesteryl ester transfer protein
Reinhard EJ, et al.
Journal of Medicinal Chemistry, 46(11), 2152-2168 (2003)
Emily J Reinhard et al.
Journal of medicinal chemistry, 46(11), 2152-2168 (2003-05-16)
A novel series of substituted N-[3-(1,1,2,2-tetrafluoroethoxy)benzyl]-N-(3-phenoxyphenyl)-trifluoro-3-amino-2-propanols is described which potently and reversibly inhibit cholesteryl ester transfer protein (CETP). Starting from the initial lead 1, various substituents were introduced into the 3-phenoxyaniline group to optimize the relative activity for inhibition of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service