657697
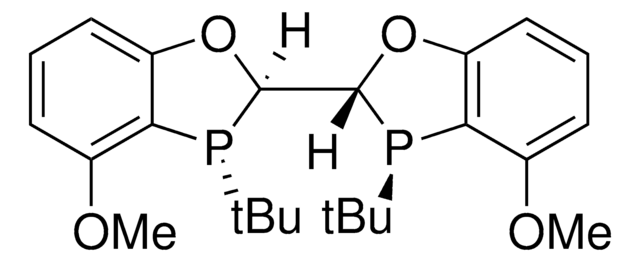
(1R,1′R,2S,2′S)-DuanPhos
Synonym(s):
(1R,1′R,2S,2′S)-2,2′-Di-tert-butyl-2,3,2′,3′-tetrahydro-1H,1′H-(1,1′)biisophosphindolyl
About This Item
Recommended Products
optical activity
[α]20/D +18°, c = 1 in chloroform
mp
214-246 °C
functional group
phosphine
SMILES string
CC(C)(C)P1Cc2ccccc2C1C3P(Cc4ccccc34)C(C)(C)C
InChI
1S/C24H32P2/c1-23(2,3)25-15-17-11-7-9-13-19(17)21(25)22-20-14-10-8-12-18(20)16-26(22)24(4,5)6/h7-14,21-22H,15-16H2,1-6H3/t21-,22-,25-,26-/m1/s1
InChI key
HCBRTCFUVLYSKU-SAZLYLDSSA-N
Related Categories
General description
Application
Catalytic ligand used for:
- Stereoselective synthesis of cyano-substituted dihydropyrroles by annulation of cyanoallenes
- Rhodium-catalyzed intermolecular enantioselective hydroacylation of alkynes to give alpha- and beta-substituted unsaturated ketones by kinetic resolution
- Stereoselective preparation of β-amino nitriles via rhodium-catalyzed asymmetric hydrogenation of amino acrylonitriles
- Stereoselective preparation of anti-1,3-amino alcohols via rhodium-catalyzed asymmetric hydrogenation of β-ketoenamide intermediates
- Stereoselective preparation of acetylaminoindane via rhodium-catalyzed asymmetric hydrogenation of acetylaminoindene
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Sigma-Aldrich has research quantities of a series of Zhang’s chiral phosphines for catalytic asymmetric hydrogenations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)

![(R)-(+)-2-[2-(Diphenylphosphino)phenyl]-4-isopropyl-2-oxazoline ≥97.0% (CHN)](/deepweb/assets/sigmaaldrich/product/structures/854/832/42ef7795-7199-4547-b48d-6fd210548e2d/640/42ef7795-7199-4547-b48d-6fd210548e2d.png)
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/168/768/54a48841-6fe6-437a-81af-8c2e54117ef3/640/54a48841-6fe6-437a-81af-8c2e54117ef3.png)

