637386
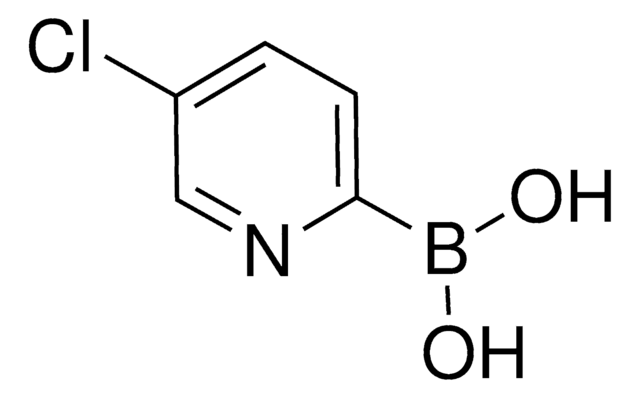
6-Chloro-3-pyridinylboronic acid
≥95.0%
Synonym(s):
2-Chloro-5-pyridineboronic acid
About This Item
Recommended Products
Assay
≥95.0%
form
solid
mp
165 °C (lit.)
functional group
chloro
SMILES string
OB(O)c1ccc(Cl)nc1
InChI
1S/C5H5BClNO2/c7-5-2-1-4(3-8-5)6(9)10/h1-3,9-10H
InChI key
WPAPNCXMYWRTTL-UHFFFAOYSA-N
Application
- To prepare biologically significant 3-arylcoumarins by reacting with 3-chlorocoumarin through Suzuki reaction.
- As a substrate in the synthesis of 11-(pyridinylphenyl)steroid with progesterone agonist/antagonist profile.
- As a substrate in the preparation of α- secondary and tertiary pyridines by the reaction of pyridotriazoles with boronic acids.
- As a substrate in the palladium-catalyzed α-arylation of saturated cyclic amines and N-methyl amines.
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service