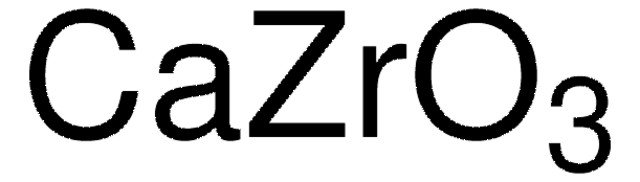633801
Calcium titanate
nanopowder, <100 nm particle size (BET), 99% trace metals basis
Synonym(s):
CALCIUM TITANIUM OXIDE
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CaTiO3
CAS Number:
Molecular Weight:
135.94
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99% trace metals basis
form
nanopowder
surface area
5-20 m2/g
particle size
<100 nm (BET)
density
4.1 g/mL at 25 °C (lit.)
SMILES string
[Ca++].[O-][Ti]([O-])=O
InChI
1S/Ca.3O.Ti/q+2;;2*-1;
InChI key
AOWKSNWVBZGMTJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Annette Bussmann-Holder
Journal of physics. Condensed matter : an Institute of Physics journal, 24(27), 273202-273202 (2012-06-22)
This article reviews the polarizability model and its applications to ferroelectric perovskite oxides. The motivation for the introduction of the model is discussed and nonlinear oxygen ion polarizability effects and their lattice dynamical implementation outlined. While a large part of
Abnormal Poisson's ratio and linear compressibility in perovskite materials.
C W Huang et al.
Advanced materials (Deerfield Beach, Fla.), 24(30), 4170-4174 (2012-05-10)
Wei Yang et al.
Environmental science & technology, 46(20), 11280-11288 (2012-09-19)
A series of LaFe(1-x)(Cu, Pd)(x)O(3-δ) perovskites was fully characterized and tested for the selective catalytic reduction (SCR) of NO by C(3)H(6) in the presence of O(2). The adsorbed species and surface reactions were investigated for mechanistic study by means of
Maria Flytzani-Stephanopoulos et al.
Annual review of chemical and biomolecular engineering, 3, 545-574 (2012-05-09)
Our aim in this review is to assess key recent findings that point to atomically dispersed noble metals as catalytic sites on solid supports, which may be viewed as ligands bonded to the metal. Both zeolites and open metal oxide
Hui-Seon Kim et al.
Scientific reports, 2, 591-591 (2012-08-23)
We report on solid-state mesoscopic heterojunction solar cells employing nanoparticles (NPs) of methyl ammonium lead iodide (CH(3)NH(3))PbI(3) as light harvesters. The perovskite NPs were produced by reaction of methylammonium iodide with PbI(2) and deposited onto a submicron-thick mesoscopic TiO(2) film
Articles
Synthesis, Properties, and Applications of Perovskite-Phase Metal Oxide Nanostructures
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service