574686
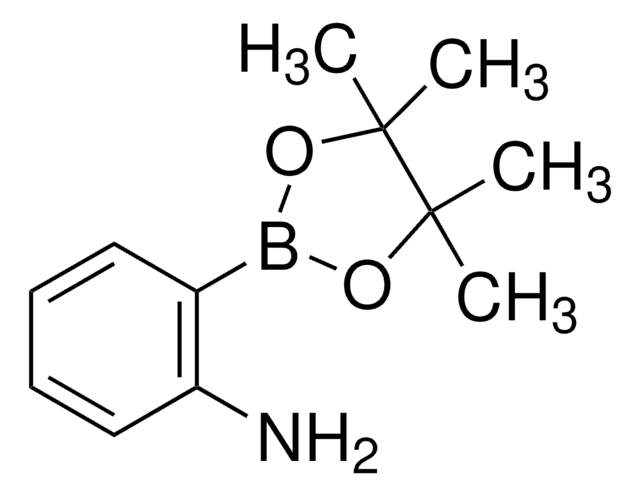
3-Aminophenylboronic acid pinacol ester
97%
Synonym(s):
3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H18BNO2
CAS Number:
Molecular Weight:
219.09
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
90-94 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2cccc(N)c2
InChI
1S/C12H18BNO2/c1-11(2)12(3,4)16-13(15-11)9-6-5-7-10(14)8-9/h5-8H,14H2,1-4H3
InChI key
YMXIIVIQLHYKOT-UHFFFAOYSA-N
Application
3-Aminophenylboronic acid pinacol ester can be used:
- As a starting material for the synthesis of 6-(hetero)arylthieno[3,2-b]pyridines, which are known to inhibit human tumor cells selectively.
- In the functionalization of deuteroporphyrin IX dimethyl ester through Suzuki-Miyaura coupling.
- To prepare benzo[d]oxazole based type-I FLT3-ITD inhibitors.
Legal Information
Product of Boron Molecular
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Functionalization of Deutero-and Protoporphyrin IX Dimethyl Esters via Palladium-Catalyzed Coupling Reactions
O?Brien JM, et al.
The Journal of Organic Chemistry, 84(10), 6158-6173 (2019)
Discovery of benzo [d] oxazole derivatives as the potent type-I FLT3-ITD inhibitors
Bao J, et al.
Bioorganic Chemistry, 94(10), 103248-103248 (2020)
Efficient synthesis of 6-(hetero) arylthieno [3, 2-b] pyridines by Suzuki-Miyaura coupling. Evaluation of growth inhibition on human tumor cell lines, SARs and effects on the cell cycle
Queiroz M-J, et al.
European Journal of Medicinal Chemistry, 45(12), 5628-5634 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service