567191
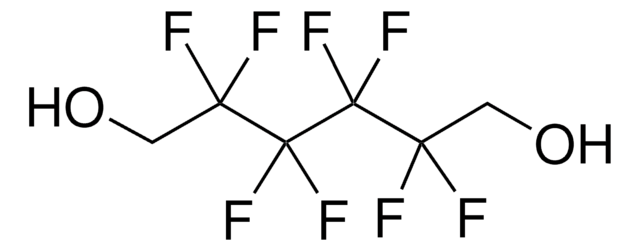
2,2,3,3-Tetrafluoro-1,4-butanediol
Synonym(s):
2,2,3,3-Tetrafluorobutanediol, NSC 95113, Tetrafluoro-1,4-butanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2CF2CF2CH2OH
CAS Number:
Molecular Weight:
162.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
bp
110-112 °C/13 mmHg (lit.)
mp
77-82 °C (lit.)
functional group
fluoro
hydroxyl
SMILES string
OCC(F)(F)C(F)(F)CO
InChI
1S/C4H6F4O2/c5-3(6,1-9)4(7,8)2-10/h9-10H,1-2H2
InChI key
CDZXJJOGDCLNKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,2,3,3-Tetrafluoro-1,4-butanediol may be used in the synthesis of the following:
- segmented polyurethanes(SPU 001-002)
- PFPE(perfluoro-polyether)modified segmented polyurethanes (SPU 003-006)
- 2,2,3,3-tetrafluorobutane-1,4-diyl dicarbamate
- 2,2,3,3-tetrafluorobutane-1,4-diyl dinitrate
- 2,2,3,3-tetrafluoro-4-((trimethylsilyl)methoxy)butanol
Reactant for:
Enzymic synthesis of silicone fluorinated aliphatic polyester amides
Enzymic synthesis of silicone fluorinated aliphatic polyester amides
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Comparison of Photocyclization Reactions of Fluoro-vs Nonfluoro-Substituted Polymethyleneoxy Donor Linked Phthalimides"
Park JH, et al.
Bulletin of the Korean Chemical Society,, 34(4), 1108-1114 (2013)
"Synthesis of nitrate ester and nitramine derivatives of polyfluoro alkyl compounds for high energy materials"
Srinivas D and Ghule.DV
Royal Society of Chemistry Advances, 6(10), 7712-7716 (2016)
"Synthesis and surface properties of environmentally responsive segmented polyurethanes"
Vaidya A and Chaudhury.KM
Journal of Colloid and Interface Science, 249(1), 235-245 (2002)
Piotr Król et al.
Colloid and polymer science, 290(10), 879-893 (2012-06-19)
The polarity of polyurethane coats was studied on the basis of the goniometric method for determination of wetting angle values, on the basis of calculated surface free energy (SFE) values by the van Oss-Good and Owens-Wendt methods, and on the
Piotr Król et al.
Colloid and polymer science, 289(15-16), 1757-1767 (2011-12-02)
WAXS, DSC and AFM methods were employed to compare phase structures of the coatings obtained from waterborne polyurethane cationomers which had been synthesised in the reaction of some diisocyanates (MDI, IPDI, TDI and HDI) with polyoxyethylene glycols (M = 600 and 2,000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service