All Photos(2)
About This Item
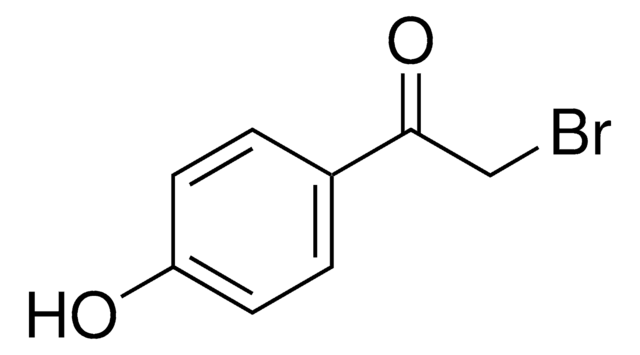
Linear Formula:
BrCH2COC6H4CN
CAS Number:
Molecular Weight:
224.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
92-96 °C (lit.)
functional group
bromo
ketone
nitrile
SMILES string
BrCC(=O)c1ccc(cc1)C#N
InChI
1S/C9H6BrNO/c10-5-9(12)8-3-1-7(6-11)2-4-8/h1-4H,5H2
InChI key
LJANCPRIUMHGJE-UHFFFAOYSA-N
General description
2-Bromo-4′-cyanoacetophenone can be synthesized from ethylbenzene via aerobic photooxidation using aqueous HBr.
Application
2-Bromo-4′-cyanoacetophenone may be used to synthesize:
- 3-acylindolizines
- (2R,3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol
- 1-[2-(4-cyanophenyl)-2-oxoethyl]-1,10-phenanthrolinium bromide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Application of DMF?methyl sulfate adduct in the regioselective synthesis of 3-acylated indolizines.
Przewloka T, et al.
Tetrahedron Letters, 48(33), 5739-5742 (2007)
Synthesis and antifungal activity of novel thiazole-containing triazole antifungals. II. Optically active ER-30346 and its derivatives.
Tsuruoka, Akihiko, et al.
Chemical & Pharmaceutical Bulletin, 46(4), 623-630 (1998)
New 1, 10-phenanthroline derivatives with potential antitumoral activity.
Dumitrascu F, et al.
Rev. Roum. Chim., 53(3), 183-187 (2008)
Norihiro Tada et al.
Organic & biomolecular chemistry, 8(20), 4701-4704 (2010-08-27)
The direct synthesis of α-bromoketones from alkylarenes by aerobic photooxidation with hydrobromic acid is reported. The key success for this direct oxidative reaction is due to control of bromination with acetic acid and ethanol, which are generated in situ by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service