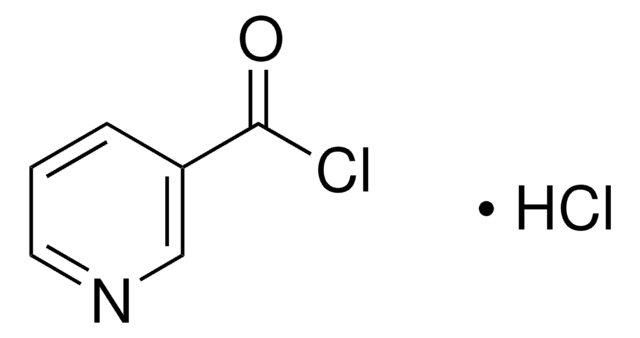
515027
4-Methyl-1-piperazinecarbonyl chloride hydrochloride
97%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H11ClN2O · HCl
CAS Number:
Molecular Weight:
199.08
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
225-228 °C (lit.)
functional group
chloro
SMILES string
Cl[H].CN1CCN(CC1)C(Cl)=O
InChI
1S/C6H11ClN2O/c1-8-2-4-9(5-3-8)6(7)10/h2-5H2,1H3
InChI key
FBAIGEMWTOSCRU-UHFFFAOYSA-N
Application
4-Methyl-1-piperazinecarbonyl chloride hydrochloride may be used in the synthesis of tert-butyl 2-(N-ethyl-4-methylpiperzaine-1-carboxamido)ethylcarbamate and 4b,8,8-trimethyl-9,10-dioxo-4b,5,6,7,8,8a,9,10-octahydrophenanthren-2-yl 4-methyl-piperazine-1-carboxylate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Qiong Wei et al.
PloS one, 7(12), e52199-e52199 (2013-01-10)
Thymidine kinases (TKs) have been considered one of the potential targets for anticancer therapeutic because of their elevated expressions in cancer cells. However, nucleobase analogs targeting TKs have shown poor selective cytotoxicity in cancer cells despite effective antiviral activity. 3'-Deoxythymidine
Amy Junnila et al.
Bioconjugate chemistry, 18(6), 1818-1823 (2007-10-12)
New fluorescein and rhodamine B-labeled antifilarial drug DEC analogues for use in drug localization studies with confocal microscopy have been prepared by a high-yield three-step synthesis. The resulting beta-amine-substituted DEC analogue has a single ethyl substituent which is beta-aminated to
Brown DJ.
The Chemistry of Heterocyclic Compounds, 317-317 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![Dibenz [b,f]azepine-5-carbonyl chloride 90%](/deepweb/assets/sigmaaldrich/product/structures/407/098/2961006c-9a3d-4aed-a20e-35b97bfa45c2/640/2961006c-9a3d-4aed-a20e-35b97bfa45c2.png)