All Photos(1)
About This Item
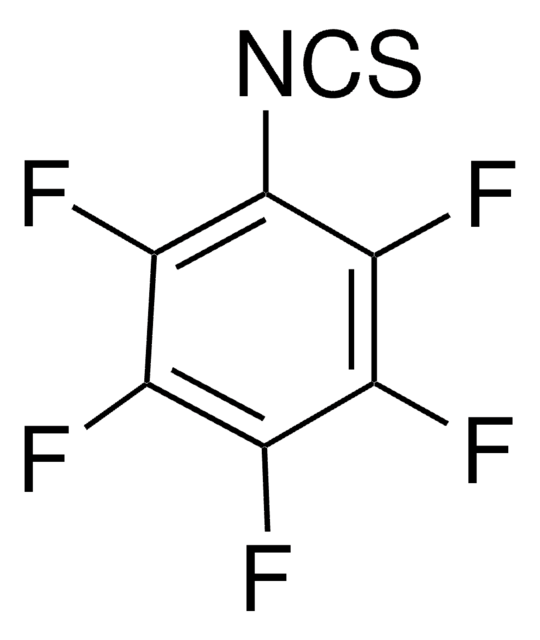
Linear Formula:
F2C6H3NCS
CAS Number:
Molecular Weight:
171.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.604 (lit.)
bp
217 °C (lit.)
density
1.325 g/mL at 25 °C (lit.)
functional group
fluoro
isothiocyanate
storage temp.
2-8°C
SMILES string
Fc1cccc(F)c1N=C=S
InChI
1S/C7H3F2NS/c8-5-2-1-3-6(9)7(5)10-4-11/h1-3H
InChI key
DBSXNGIBAKYMSS-UHFFFAOYSA-N
General description
2,6-Difluorophenyl isothiocyanate (1,3-Difluoro-2-isothiocyanatobenzene), an aryl isocyanate, can be prepared from difluoroaniline. 1H-[1,2,4]triazole-3,5-diamine undergoes acylation with 2,6-difluorophenyl isothiocyanate to form the corresponding 1-acyl-1H-[1,2,4]triazole-3,5-diamine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J W Tilley et al.
Journal of medicinal chemistry, 23(12), 1387-1392 (1980-12-01)
Structure-activity studies were carried out on a series of antihypertensive 1-(2-aminoethyl)-3-(substituted phenyl)thioureas. From this class of compounds, the 2,6-dichlorophenyl analogue 2 was found to have potent oral antihypertensive activity in two hypertensive rat models and the renal hypertensive dog. In
1-Acyl-1H-[1,2,4] triazole-3,5-diamine Analogues as Novel and Potent Anticancer Cyclin-Dependent Kinase Inhibitors: Synthesis and Evaluation of Biological Activities.
Lin R, et al.
Journal of Medicinal Chemistry, 48(13), 4208-4211 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service