All Photos(1)
About This Item
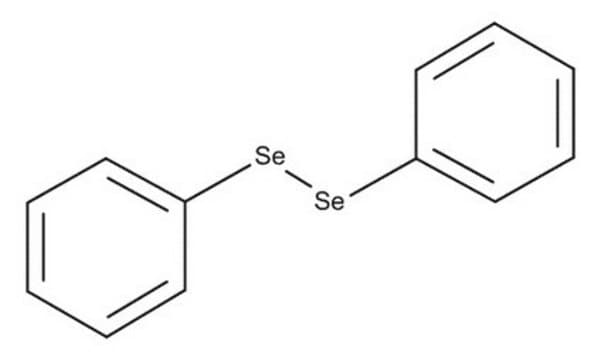
Linear Formula:
(C6H5)2Se
CAS Number:
Molecular Weight:
233.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.6465 (lit.)
bp
115-117 °C (lit.)
density
1.338 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[Se](c1ccccc1)c2ccccc2
InChI
1S/C12H10Se/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h1-10H
InChI key
ORQWTLCYLDRDHK-UHFFFAOYSA-N
Related Categories
General description
Diphenyl selenide is an organoselenium compound. Its standard enthalpies of combustion, formation and the mean bond-dissociation energy have been calculated. Its potential as a neutral carrier to develop silver-selective membrane electrode has been investigated. Diphenyl selenide undergoes oxidation to form selenoxides on treating with N-bromosuccinimide followed by alkaline hydrolysis.
Application
Used for Anti-selective Michael addition of thiols and their analogs to nitro-olefins.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Organoselenide as a novel ionophore for a silver-selective membrane electrode.
Katsu T and Xu D.
Analytical Letters, 31(12), 1979-1989 (1998)
Oxidation of selenides and tellurides with positive halogenating species.
Detty MR
The Journal of Organic Chemistry, 45(2), 274-279 (1980)
Xinghua Dong et al.
ACS nano, 14(5), 5400-5416 (2020-04-24)
Radiotherapy (RT) in practical use often suffers from off-target side effects and ineffectiveness against hypoxic tumor microenvironment (TME) as well as remote metastases. With regard to these problems, herein, we provide semiconductor heterojunction structured WO2.9-WSe2-PEG nanoparticles to realize a synergistic
Enthalpies of combustion of selenium and diphenyl selenide.
Barnes DS and Mortimer CT.
The Journal of Chemical Thermodynamics, 5(3), 371-377 (1973)
The Journal of Organic Chemistry, 55, 2437-2437 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service