All Photos(1)
About This Item
Linear Formula:
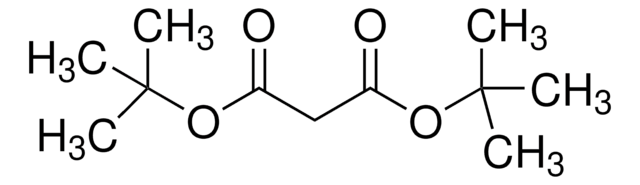
H2C=CHCH2CH(CO2CH3)2
CAS Number:
Molecular Weight:
172.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.435 (lit.)
bp
207 °C/771 mmHg (lit.)
density
1.071 g/mL at 25 °C (lit.)
functional group
allyl
ester
SMILES string
COC(=O)C(CC=C)C(=O)OC
InChI
1S/C8H12O4/c1-4-5-6(7(9)11-2)8(10)12-3/h4,6H,1,5H2,2-3H3
InChI key
VZNFVLWVVHHMBG-UHFFFAOYSA-N
Related Categories
Application
Dimethyl allylmalonate may be used in the preparation of:
- terminal alkyl silane
- diallyl malonates
- bromosulfoxide
- dimethyl-2-allyl-2-{4-methyl-2-[(tetrahydro-2H-pyran-2-yloxy)methyl]deca-2,3-dienoyl}malonate
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tandem cyclization/hydrosilylation of functionalized 1, 6-dienes catalyzed by a cationic palladium complex.
Widenhoefer RA and DeCarli MA.
Journal of the American Chemical Society, 120(15), 3805-3806 (1998)
Synthesis of substituted cyclopentenes from the Baylis-Hillman adducts via ring-closing metathesis reaction.
Lee KY, et al.
Bull. Korean Chem. Soc., 25(8), 1280-1282 (2004)
The study of intramolecular free radical cyclizations of a-sulfonyl and a-sulfinyl radicals.
Yeun-Min T, et al.
Tetrahedron Letters, 31(42), 6047-6050 (1990)
Krause N.
Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, 138-138 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service