442178
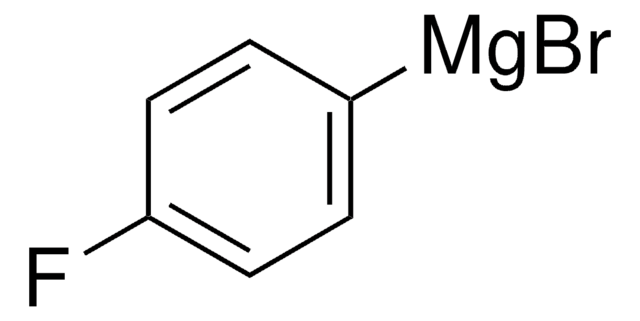
p-Tolylmagnesium bromide solution
1.0 M in THF
Synonym(s):
4-Methylphenylmagnesium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H4MgBr
CAS Number:
Molecular Weight:
195.34
Beilstein:
636491
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
1.0 M in THF
bp
65-67 °C
density
1.002 g/mL at 25 °C
SMILES string
Cc1ccc([Mg]Br)cc1
InChI
1S/C7H7.BrH.Mg/c1-7-5-3-2-4-6-7;;/h3-6H,1H3;1H;/q;;+1/p-1
InChI key
ZRJNGFJIBZKXTP-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
p-Tolylmagnesium bromide is a common Grignard reagent. It can also be used in a variety of cross-coupling reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F
Flash Point(C)
-17 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dinuclear Iron Complex-Catalyzed Cross-Coupling of Primary Alkyl Fluorides with Aryl Grignard Reagents.
Mo Z, et al.
Organometallics, 31(18), 6518-6521 (2012)
Palladium-catalyzed cross coupling of Grignard reagents with in situ-derived enol phosphates.
Miller JA
Tetrahedron Letters, 43(39), 7111-7114 (2002)
Palladium-and Nickel-Catalyzed Kumada Cross-Coupling Reactions of gem-Difluoroalkenes and Monofluoroalkenes with Grignard Reagents.
Dai W
The Journal of Organic Chemistry, 79(21), 10537-10546 (2014)
Takuji Hatakeyama et al.
Journal of the American Chemical Society, 131(33), 11949-11963 (2009-07-31)
Combinations of N-heterocyclic carbenes (NHCs) and fluoride salts of the iron-group metals (Fe, Co, and Ni) have been shown to be excellent catalysts for the cross-coupling reactions of aryl Grignard reagents (Ar(1)MgBr) with aryl and heteroaryl halides (Ar(2)X) to give
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service