434191
Vinyl bromide solution
1.0 M in THF
Synonym(s):
Bromoethylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
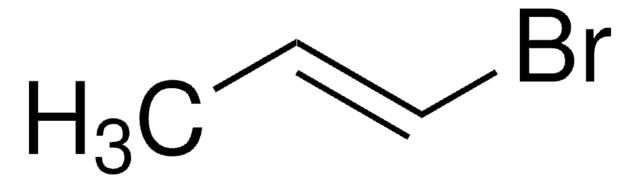
CH2=CHBr
CAS Number:
Molecular Weight:
106.95
Beilstein:
1361370
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor pressure
12.46 psi ( 55 °C)
3.6 psi ( 20 °C)
Quality Level
form
liquid
concentration
1.0 M in THF
density
0.927 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
BrC=C
InChI
1S/C2H3Br/c1-2-3/h2H,1H2
InChI key
INLLPKCGLOXCIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Vinyl bromide solution belongs to haloalkenes andis highly reactive due to the presence of an unsaturated vinyl group. It can provide flame retardancy to polymers or materials when incorporated into their structure. It is also a versatile building block for polymerization, addition reactions, substitution reactions, and cross-coupling reactions like Suzuki-Miyaura and Negishi reactions. It can be used to introduce radiolabel into molecules for medical imaging.
Application
- Sequential Vinyl Radical Cyclization/Fixation of Carbon Dioxide through Electrochemical Reduction of Vinyl Bromide in the Presence of an Electron-Transfer Mediator: This study explores the electrochemical reduction of vinyl bromide with a focus on vinyl radical cyclization and carbon dioxide fixation (A Katayama, H Senboku, 2016).
- A Comparison of the Wavelength-Dependent Photochemical Reactions of Ozone with Vinyl Bromide and Fluoride in Argon Matrices: The study compares the photochemical reactions of vinyl bromide and fluoride with ozone, examining their behavior in argon matrices (BS Ault, 2021).
Vinyl bromide solution can be used as a precursor for stereoselective synthesis of chiral 2-vinyl-tetrahydronaphthalens via asymmetric reductive coupling. These chiral compounds are valuable building blocks for natural products, agrochemicals, and liquid crystals.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nickel/Copper Co-catalyzed Enantioselective Reductive Coupling of Oxabenzonorbornadienes with Vinyl Bromides
Yao Deng, et al.
advanced synthesis and catalysis, 365, 3265-3270 (2023)
Hsin-Lun Kao et al.
Organic letters, 13(19), 5204-5207 (2011-09-02)
The synthesis of vinyl sulfides through the coupling reaction of thiols with vinyl iodides, bromides, and chlorides is described. The thiols can couple with aryl iodides in the presence of only 0.5 mol % Cu(2)O without the need for an
Piotr Pawluć et al.
Organic letters, 11(15), 3390-3393 (2009-07-04)
A new, efficient protocol for the highly stereoselective one-pot synthesis of (E)-beta-aryl vinyl iodides and (E)-beta-aryl vinyl bromides from styrenes based on sequential ruthenium-catalyzed silylative coupling-N-halosuccinimide-mediated halodesilylation reactions is reported.
Qiwu Zhao et al.
Organic letters, 10(18), 4037-4040 (2008-08-30)
A general and highly efficient synthesis of 4-alkylidene-2-azetidinones was achieved by the Cu(I)-catalyzed intramolecular C-N coupling of amides with vinyl bromides. This 4-exo ring closure was found to be fundamentally preferred over other modes (5-exo, 6-exo, and 6-endo) of cyclization
Changhui Sun et al.
Organic letters, 11(18), 4084-4087 (2009-08-20)
With the catalysis of CuI/trans-N,N'-dimethylcyclohexane-1,2-diamine, a number of carboxylic acids underwent efficient intramolecular O-vinylation with vinyl bromides leading to the synthesis of the corresponding five- and six-membered enol lactones. The same catalytic system also led to the efficient cycloisomerization of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service