405280
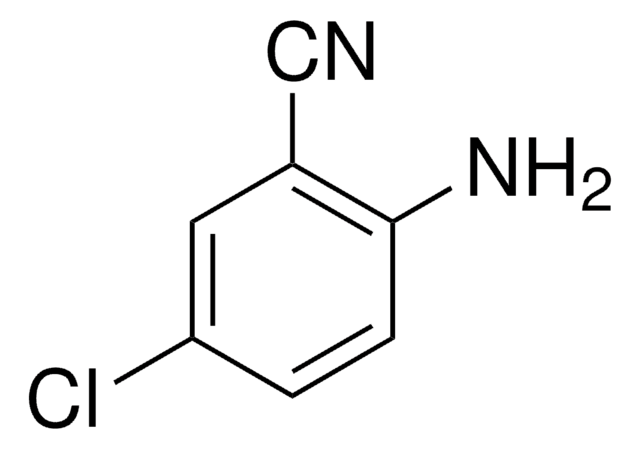
2-Amino-4-chlorobenzonitrile
99%
Synonym(s):
4-Chloroanthranilonitrile, 5-Chloro-2-cyanoaniline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H3(Cl)CN
CAS Number:
Molecular Weight:
152.58
Beilstein:
2085559
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
157-162 °C (lit.)
functional group
chloro
nitrile
SMILES string
Nc1cc(Cl)ccc1C#N
InChI
1S/C7H5ClN2/c8-6-2-1-5(4-9)7(10)3-6/h1-3H,10H2
InChI key
UZHALXIAWJOLLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-4-chlorobenzonitrile (2A4CBN) is a benzonitrile derivative. A study of the molecular structure, vibrational spectra and NBO analysis of 2A4CBN has been undertaken. Transformation of 2A4CBN into quinazoline-2,4(1H,3H)-diones at atmospheric pressure of CO2 in the presence of monomeric tungstate, TBA2[WO4] (TBA = tetra-n-butylammonium) has been reported.
Application
2-Amino-4-chlorobenzonitrile may be used in the preparation of the following:
- 6-chloro-4-(1-diethylamino-4-pentylamino)-2-(p-methoxyphenyl)-quinazoline dihydrochloride
- 7-chloro-4-(1-diethylamino-4-pentylamino)-2-(p-methoxyphenyl)-quinazoline dihydrochloride
- 4,7-dichloro-2-(2-methylprop-1-enyl)-6-nitroquinazoline
- 6-chlorotacrine
- N-(2-chloro-5-cyanophenyl)-4,4,4-trifluorobutanamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Youssef Kabri et al.
Molecules (Basel, Switzerland), 15(5), 2949-2961 (2010-07-27)
New diarylquinazolines displaying pharmaceutical potential were synthesized in high yields from 4,7-dichloro-2-(2-methylprop-1-enyl)-6-nitroquinazoline by using microwave-promoted regioselective Suzuki-Miyaura cross-coupling reactions.
A novel hybrid of 6-chlorotacrine and metal-amyloid-β modulator for inhibition of acetylcholinesterase and metal-induced amyloid-β aggregation.
Kochi A, et al.
Chemical Science, 4(11), 4137-4145 (2013)
FT-IR and FT-Raman spectra, vibrational assignments, NBO analysis and DFT calculations of 2-amino-4-chlorobenzonitrile.
Sudha S, et al.
Journal of Molecular Structure, 958(2), 148-156 (2011)
Regioselective Suzuki-Miyaura reaction: application to the microwave-promoted synthesis of 4,7-diarylquinazolines
Kabri, Y.; et al.
Molecules (Basel), 15(5), 2949-2961 (2010)
6-(and 7-)-Chloro-4-(1-diethylamino-4-pentylamino)-2-(p-methoxyphenyl)-quinazoline Dihydrochlorides.
McKee RL, et al.
Journal of the American Chemical Society, 69(4), 940-942 (1947)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service