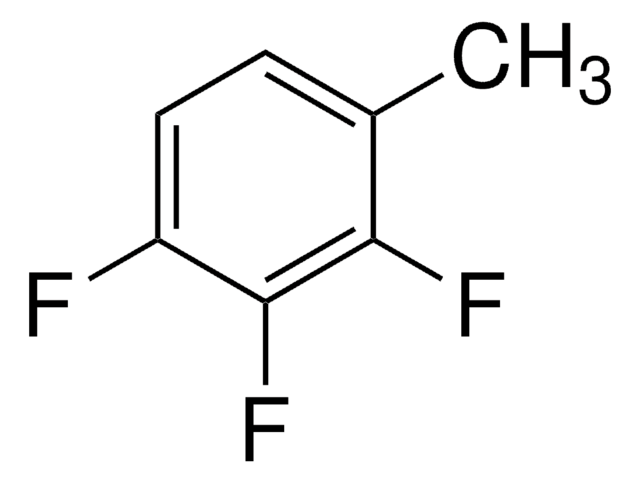
381438
Benzaldehyde dimethyl acetal
95%
Synonym(s):
α,α-Dimethoxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH(OCH3)2
CAS Number:
Molecular Weight:
152.19
Beilstein:
2044501
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.493 (lit.)
bp
87-89 °C/18 mmHg (lit.)
density
1.014 g/mL at 25 °C (lit.)
functional group
acetal
ether
phenyl
SMILES string
COC(OC)c1ccccc1
InChI
1S/C9H12O2/c1-10-9(11-2)8-6-4-3-5-7-8/h3-7,9H,1-2H3
InChI key
HEVMDQBCAHEHDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzaldehyde dimethyl acetal is an organic building block. Pyridinium tosylate-catalyzed acetal exchange reaction between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-α-D-glucofuranose is reported to afford 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranose. The kinetics of the hydrolysis of benzaldehyde dimethyl acetal over amberlite IR-120 has been studied using a circulated batch reactor in dioxane. One-pot tandem conversion of benzaldehydedimethylacetal to trans-1-nitro-2-phenylethylene has been reported.
Application
Benzaldehyde dimethyl acetal is suitable for use in the synthesis of 4,6-dihydroxy sugar, required for the total synthesis of Porphyromonas gingivalis 381 derived lipid A. It may be used in the preparation of 1-O-methyl-2,3-di-O-galloyl-β-D-glucose.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Akerfeldt et al.
Carbohydrate research, 158, 137-145 (1986-12-15)
Pyridinium tosylate-catalyzed acetal exchange between benzaldehyde dimethyl acetal and 6-O-(tert-butyldiphenylsilyl)-1,2-O-isopropylidene-alpha-D-glucofu ranose was investigated as an alternative to the original procedure of Brigl and Grüner (condensation of a D-glucose triol with benzaldehyde under zinc halide catalysis) for synthesis of 3,5-O-benzylidene-1,2-O-isopropylidene-alpha-D-glucofuranose. The
T Ogawa et al.
FEMS immunology and medical microbiology, 28(4), 273-281 (2000-07-13)
A synthetic lipid A of Porphyromonas gingivalis strain 381 (compound PG-381), which is similar to its natural lipid A, demonstrated no or very low endotoxic activities as compared to Escherichia coli-type synthetic lipid A (compound 506). On the other hand
Mesoporous silica with site-isolated amine and phosphotungstic acid groups: a solid catalyst with tunable antagonistic functions for one-pot tandem reactions.
N Raveendran Shiju et al.
Angewandte Chemie (International ed. in English), 50(41), 9615-9619 (2011-09-16)
M Toda et al.
Bioscience, biotechnology, and biochemistry, 65(3), 542-547 (2001-05-02)
The clove ellagitannins and their related polygalloyl-glucoses inhibited maltase activity of rat intestinal alpha-glucosidases. The structure-activity relationship study of those galloylglucoses, varying the extent of galloylation on the glucose core, with the ellagitannins, indicated that an increasing number of galloyl
Kinetics of hydrolysis of benzaldehyde dimethyl acetal over Amberlite IR-120.
Altiokka MR and Hosgun HL.
Industrial & Engineering Chemistry Research, 46(4), 1058-1062 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service