All Photos(2)
About This Item
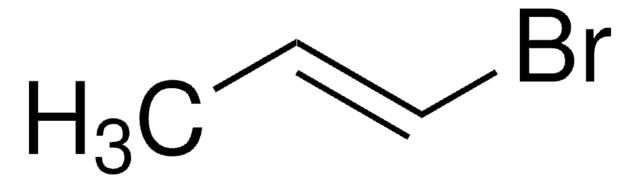
Linear Formula:
(CH3)2C=C(Br)CH3
CAS Number:
Molecular Weight:
149.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
contains
copper pellets as stabilizer
refractive index
n20/D 1.474 (lit.)
bp
40 °C/75 mmHg (lit.)
density
1.284 g/mL at 25 °C (lit.)
functional group
alkyl halide
bromo
SMILES string
C\C(C)=C(\C)Br
InChI
1S/C5H9Br/c1-4(2)5(3)6/h1-3H3
InChI key
DBELOSOZLGEZBM-UHFFFAOYSA-N
General description
2-Bromo-3-methyl-2-butene is a vinylic bromide compound. Palladium/di-1-adamantyl-n-butylphosphine-catalyzed reductive carbonylation of 2-bromo-3-methyl-2-butene has been reported. Cross-coupling reaction of 2-bromo-3-methyl-2-butene with potassium 6-(benzoyloxy)hexyltrifluoroborate and 3-(benzoyloxy)propyltrifluoroborate has been investigated.
Application
2-Bromo-3-methyl-2-butene may be used in the preparation of:
- 2,3,4,5-tetramethyl-2,4-hexadiene
- 2-iodo-3-methyl-2-butene
- diastereomers of 2-amino-3-hydroxy-4,5-dimethylhexanoic acid
- lithium reagent, 2-lithio-3-methylbut-2-ene
- D-allo-(2R,3R,4R)-2-amino-3-hydroxy-4,5-dimethylhexanoic acid-containing peptide, pipecolidepsin A.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
77.0 °F
Flash Point(C)
25 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of All the Diastereomers of 2-Amino-3-hydroxy-4, 5-dimethylhexanoic Acid.
Spengler J and Albericio F.
European Journal of Organic Chemistry, 1, 44-47 (2014)
Marta Pelay-Gimeno et al.
Nature communications, 4, 2352-2352 (2013-08-31)
Pipecolidepsin A is a head-to-side-chain cyclodepsipeptide isolated from the marine sponge Homophymia lamellosa. This compound shows relevant cytotoxic activity in three human tumour cell lines and has unique structural features, with an abundance of non-proteinogenic residues, including several intriguing amino
Synthesis and Molecular Structure of 2, 3, 4, 5-Tetramethyl-2, 4-hexadiene.
Br M, et al.
Acta Chemica Scandinavica. Series B, 31, 387-390 (1977)
Palladium/di-1-adamantyl-n-butylphosphine-catalyzed reductive carbonylation of aryl and vinyl halides.
Brennfuhrer A, et al.
Tetrahedron, 63(27), 6252-6258 (2007)
Artis Klapars et al.
Journal of the American Chemical Society, 124(50), 14844-14845 (2002-12-12)
A mild and general method for the conversion of aryl, heteroaryl, and vinyl bromides into the corresponding iodides was developed utilizing a catalyst system comprising 5 mol % of CuI and 10 mol % of a 1,2- or 1,3-diamine ligand.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service