308323
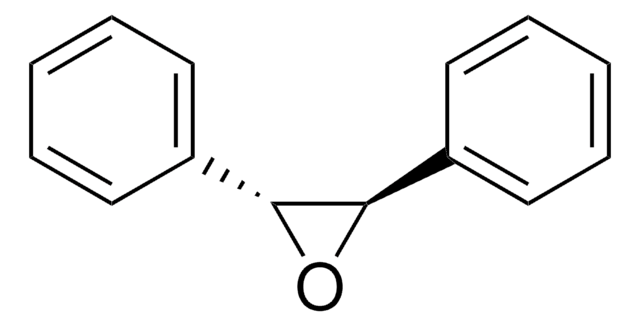
cis-Stilbene oxide
97%
Synonym(s):
cis-2,3-Diphenyloxirane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12O
CAS Number:
Molecular Weight:
196.24
Beilstein:
82737
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
mp
38-40 °C (lit.)
storage temp.
2-8°C
SMILES string
O1[C@H]([C@H]1c2ccccc2)c3ccccc3
InChI
1S/C14H12O/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14+
InChI key
ARCJQKUWGAZPFX-OKILXGFUSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Paloma Vidal et al.
The Journal of organic chemistry, 72(9), 3166-3170 (2007-03-10)
This study presents a simple method for measuring long-range heteronuclear coupling constants between protons and proton-bearing carbons. The approach involves recording two conventional 1D-TOCSY experiments in which the offset of the selective proton pulse is set on the low- and
Yongping Zhang et al.
Journal of chromatography. A, 1191(1-2), 188-192 (2007-12-07)
A facile strategy based on click chemistry for preparation of the structurally well-defined native beta-cyclodextrin (beta-CD) based chiral stationary phase (CSP) was proposed. The beta-CD CSP was evaluated by enatioseparation of benzoin, trans-stilbene oxide, Troger's base, bendroflumethiazide, ketoprofen, chlorthalidone, three
Richard Lonsdale et al.
Biochemistry, 51(8), 1774-1786 (2012-01-28)
Soluble epoxide hydrolase (sEH) is an enzyme involved in drug metabolism that catalyzes the hydrolysis of epoxides to form their corresponding diols. sEH has a broad substrate range and shows high regio- and enantioselectivity for nucleophilic ring opening by Asp333.
S Bernardini et al.
Mutagenesis, 16(3), 277-281 (2001-04-26)
About 50% and 15% of Caucasians lack the glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genes and the corresponding enzyme activity, respectively. Both of these polymorphisms have been shown to affect the genotoxicity of some epoxides in cultured human lymphocytes.
Kouhei Shimomura et al.
Nature chemistry, 6(5), 429-434 (2014-04-24)
In the chromatographic separation of enantiomers the order of elution is determined by the strength of diasteromeric interactions between the components of the mixture and a chiral stationary phase. For analytical purposes, it is ideal to have the minor component
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service