283754
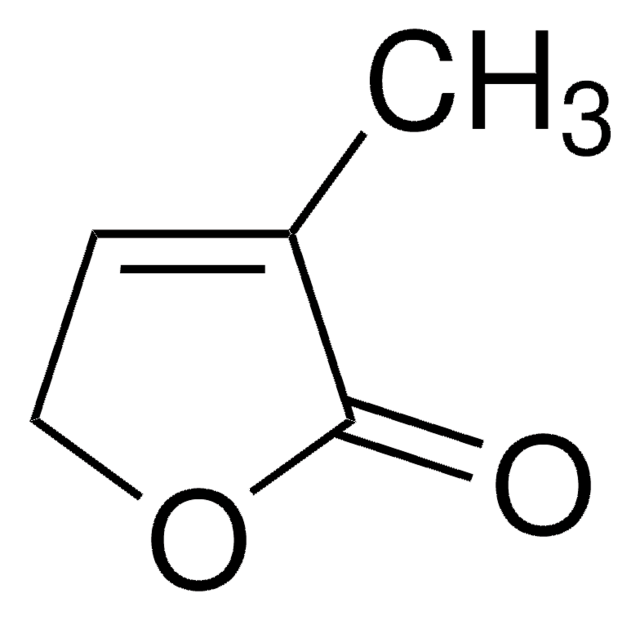
2(5H)-Furanone
98%
Synonym(s):
γ-Crotonolactone, 2-Buten-1,4-olide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4O2
CAS Number:
Molecular Weight:
84.07
Beilstein:
383585
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
86-87 °C/12 mmHg (lit.)
mp
4-5 °C (lit.)
density
1.185 g/mL at 25 °C (lit.)
functional group
ester
storage temp.
2-8°C
SMILES string
O=C1OCC=C1
InChI
1S/C4H4O2/c5-4-2-1-3-6-4/h1-2H,3H2
InChI key
VIHAEDVKXSOUAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Chiral urea compounds catalyzed hetero-Michael addition reaction of 2(5H)-furanone (γ-crotonolactone) to pyrrolidine. The quorum sensing inhibition activity by 2(5H)-furanone was studied using bioindicator strains.
Application
2(5H)-Furanone (γ-Crotonolactone) has been used in:
- synthesis of (+)-L-733,060, (+)-CP-99,994 and (2S,3R)-3-hydroxypipecolic acid
- synthesis of 5-substituted 2(5H) furanones (γ-butenolides) via direct aldol reaction with aromatic aldehydes catalyzed by bifunctional aminothiourea and aminosquaramide organocatalysts
- Michael addition reactions for synthesis of lignans
- three-component Michael-Aldol reactions with an aldehyde anda thiolate or carbanion
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron, 49, 9039-9039 (1993)
Tetrahedron, 49, 8073-8073 (1993)
Yoshihiro Sohtome et al.
Chemical & pharmaceutical bulletin, 52(4), 477-480 (2004-04-02)
Chiral urea compounds 10a-g were synthesized as catalysts for conjugate addition of pyrrolidine (2) to gamma-crotonolactone (3). In the presence of a catalytic amount of the chiral ureas, this hetero-Michael reaction was greatly accelerated. Asymmetric induction was observed with the
Tetrahedron, 49, 4173-4173 (1993)
Rebecca J Chesterfield et al.
ACS synthetic biology, 9(8), 2107-2118 (2020-08-14)
Strigolactones are plant hormones and rhizosphere signaling molecules with key roles in plant development, mycorrhizal fungal symbioses, and plant parasitism. Currently, sensitive, specific, and high-throughput methods of detecting strigolactones are limited. Here, we developed genetically encoded fluorescent strigolactone biosensors based
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service