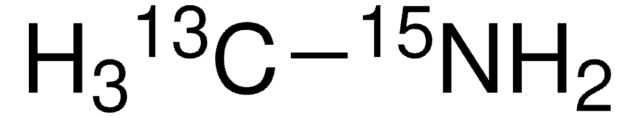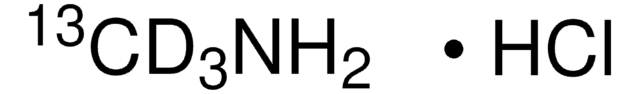All Photos(1)
About This Item
Linear Formula:
13CH3NH2 · HCl
CAS Number:
Molecular Weight:
68.51
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.12
Recommended Products
isotopic purity
99 atom % 13C
Quality Level
form
solid
technique(s)
protein expression: suitable
mp
232-234 °C (lit.)
mass shift
M+1
SMILES string
Cl.[13CH3]N
InChI
1S/CH5N.ClH/c1-2;/h2H2,1H3;1H/i1+1;
InChI key
NQMRYBIKMRVZLB-YTBWXGASSA-N
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sheeza Khan et al.
Protein and peptide letters, 20(1), 61-70 (2012-06-08)
Kidney cells of animals including human and marine invertebrates contain high amount of the protein denaturant, urea. Methylamine osmolytes are generally believed to offset the harmful effects of urea on proteins in vitro and in vivo. In this study we
Nicolas Fleury-Brégeot et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(31), 9564-9570 (2012-07-07)
Ammoniomethyl trifluoroborates are very powerful reagents that can be used to access biologically relevant aryl- and heteroaryl-methylamine motifs via Suzuki-Miyaura cross-couplings. Until now, this method was limited to the production of tertiary and primary amines. The synthesis of a large
N Cecilia Martinez-Gomez et al.
Journal of bacteriology, 195(10), 2359-2367 (2013-03-19)
The methylotroph Methylobacterium extorquens AM1 oxidizes methanol and methylamine to formaldehyde and subsequently to formate, an intermediate that serves as the branch point between assimilation (formation of biomass) and dissimilation (oxidation to CO₂). The oxidation of formaldehyde to formate is
Frank Weinhold
Journal of computational chemistry, 33(30), 2440-2449 (2012-07-28)
We have developed a "Natural Bond Critical Point" (NBCP) module for the natural bond orbital (NBO) program that allows mutual analysis of NBO-based versus Bader-type quantum theory of atoms in molecules (QTAIM) topological descriptors of chemical bonding interactions. Conventional QTAIM
Yongqian Zhang et al.
Analytica chimica acta, 752, 106-111 (2012-10-30)
Both endogenous and exogenous methylamine have been found to be involved in many human disorders. The quantitative assessment of methylamine has drawn considerable interest in recent years. Although there have been many papers about the determination of methylamine, only a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service