274984
18-Crown-6
≥99.0%
Synonym(s):
1,4,7,10,13,16-Hexaoxacyclooctadecane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H24O6
CAS Number:
Molecular Weight:
264.32
Beilstein:
1619616
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0%
form
solid
mp
42-45 °C (lit.)
functional group
ether
SMILES string
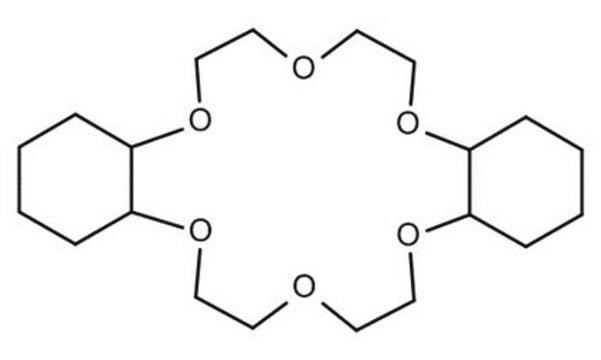
O1CCOCCOCCOCCOCCOCC1
InChI
1S/C12H24O6/c1-2-14-5-6-16-9-10-18-12-11-17-8-7-15-4-3-13-1/h1-12H2
InChI key
XEZNGIUYQVAUSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
18-Crown-6 is a macrocyclic polyether used to synthesize ionic liquid based crown-ether coordination compounds.
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Application
18-Crown-6 can be used as a catalyst for:
- N-alkylation of heterocyclic compounds in the presence of tert-butoxide base.
- Allylation of aldehydes to corresponding homoallylic alcohols using potassium allyltrifluoroborate.
- Preparation of N-propargylpyrrole by the reaction of pyrrole with potassium hydroxide.
- Polymerization of methacrylic esters and hindered alkyl acrylates.
- Chemoselective reduction of fused tetrazoles with NaBH4 and potassium hydroxide.
18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Can be useful as phase-transfer catalysts.
Other Notes
Macrocyclic polyethers with repeating (-CH2CH2O) units. The compounds are ionophoric (form stable complexes with cations).
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Improved synthesis and efficient chemoselective reduction of fused tetrazoles under phase-transfer conditions
Desai ND and Shah RD
Synthesis, 2006(19), 3275-3279 (2006)
Principles and synthetic applications in crown ether chemistry
Gokel, George W and Durst, H Dupont
Synthesis, 1976(03), 168-184 (1976)
Magnetic blocking at 10 K and a dipolar-mediated avalanche in salts of the bis (?8-cyclooctatetraenide) complex [Er (COT) 2]?.
Meihaus K R, et al.
Journal of the American Chemical Society, 135(47), 17952-17957 (2013)
Gokel, G.W.
Crown Ethers and Cryptands (1991)
18-Crown-6
Liotta, Charles, L and Berkner, Joachim
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service