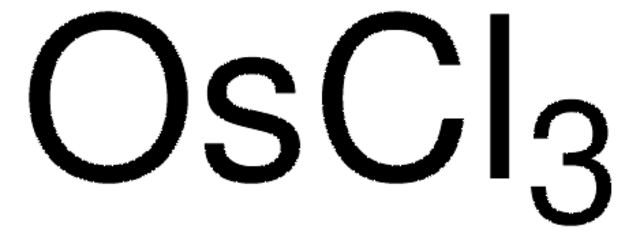All Photos(2)
About This Item
Empirical Formula (Hill Notation):
Os
CAS Number:
Molecular Weight:
190.23
EC Number:
MDL number:
UNSPSC Code:
11101711
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.9% trace metals basis
form
powder
resistivity
8.12 μΩ-cm, 20°C
bp
5027 °C (lit.)
mp
3045 °C (lit.)
density
22.61 g/cm3 (lit.)
SMILES string
[Os]
InChI
1S/Os
InChI key
SYQBFIAQOQZEGI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kilian Muñiz
Chemical Society reviews, 33(3), 166-174 (2004-03-18)
Imido complexes of osmium tetroxide are versatile compounds for olefin functionalisation. This tutorial review offers a brief historical overview on these compounds and discusses the electronic properties and reactivities of isolated imido osmium compounds in what had been the original
Isolda Romero-Canelón et al.
Journal of medicinal chemistry, 56(3), 1291-1300 (2013-02-02)
Organometallic half-sandwich complexes [M(p-cymene)(azo/imino-pyridine)X](+) where M = Ru(II) or Os(II) and X ═ Cl or I, exhibit potent antiproliferative activity toward a range of cancer cells. Not only are the iodido complexes more potent than the chlorido analogues, but they
Masruri et al.
The Journal of organic chemistry, 77(19), 8480-8491 (2012-09-12)
N-(4-toluenesulfonyloxy)carbamates based on a range of common amine protecting groups serve as preformed nitrogen sources in the intermolecular osmium-catalyzed oxyamination reaction of a variety of mono-, di-, and trisubstituted alkenes. The reactions occur with low catalyst loadings and good yields
Hideki Sugimoto et al.
Inorganic chemistry, 52(2), 543-545 (2013-01-01)
Oxidation of the hydroxoosmium(III) complex resulted in C-H bond activation of the methyl group of the supporting ligand (N,N'-dimethyl-2,11-diaza[3.3](2,6)pyridinophane). The product was an osmium(IV) complex exhibiting a seven-coordinate structure with an additional Os-CH(2) bond.
Michael H Stewart et al.
ACS nano, 6(6), 5330-5347 (2012-06-08)
The ability of luminescent semiconductor quantum dots (QDs) to engage in diverse energy transfer processes with organic dyes, light-harvesting proteins, metal complexes, and redox-active labels continues to stimulate interest in developing them for biosensing and light-harvesting applications. Within biosensing configurations
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service