All Photos(1)
About This Item
Empirical Formula (Hill Notation):
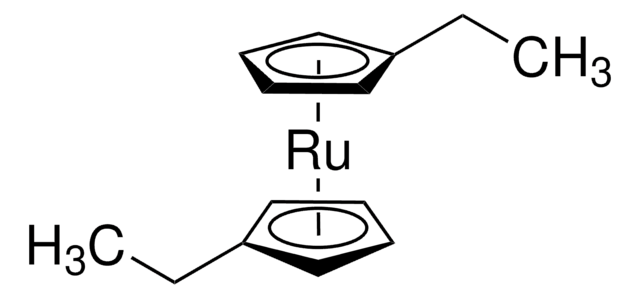
C10H10Ru
CAS Number:
Molecular Weight:
231.26
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
mp
199-201 °C (lit.)
SMILES string
[Ru].[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2
InChI
1S/2C5H5.Ru/c2*1-2-4-5-3-1;/h2*1-5H;
InChI key
BKEJVRMLCVMJLG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karin Schlotter et al.
Journal of medicinal chemistry, 48(11), 3696-3699 (2005-05-27)
Metallocene-derived bioisosteres lead to exceptionally strong binding G-protein-coupled receptor ligands, indicating substantial plasticity of the receptor excluded volume. Novel binding profiles of ferrocenylcarboxamides combining subnanomolar Ki values for the dopamine D4 receptor (1a, 0.52 nM; 1b, 0.63 nM) with superpotent
M Wenzl et al.
International journal of radiation applications and instrumentation. Part A, Applied radiation and isotopes, 39(10), 1023-1027 (1988-01-01)
Ruthenocene amphetamine analogues have the same brain uptake as iodo-labelled amphetamines. This paper compares the organ-distribution of 103Ru labelled ruthenocene- or ferrocene-amphetamine analogues in mice and rats with the same amphetamine in which H-atoms were partly substituted by D-atoms. The
Annika Gross et al.
Dalton transactions (Cambridge, England : 2003), 40(6), 1382-1386 (2010-12-25)
Organometallic conjugates of receptor-targeting peptides are proposed as interesting candidates for novel cancer therapies since they are capable of targeting a specific kind of cell. Here, we have synthesised a dicobalt hexacarbonyl alkyne compound linked to the neurotensin peptide hormone.
Damian Plażuk et al.
Organic & biomolecular chemistry, 9(2), 408-417 (2010-10-23)
(D)-Biotin was used for Friedel-Crafts acylation of electron-rich aromatic molecules--ferrocene, ruthenocene and pyrene. The reaction carried out in the presence of trifluoroacetic anhydride and trifluoromethanesulfonic acid afforded the corresponding biotinylarenes in moderate yields. These compounds, although lacking an amide bond
[Metabolism of 103 Ru-ruthenocene--in vitro experiments and a possible in vivo test for hydroxylases].
M Wenzel et al.
The International journal of applied radiation and isotopes, 32(11), 797-802 (1981-11-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service