All Photos(2)
About This Item
Empirical Formula (Hill Notation):
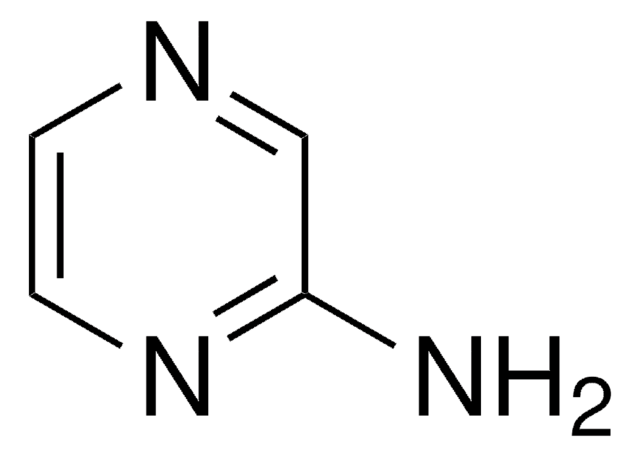
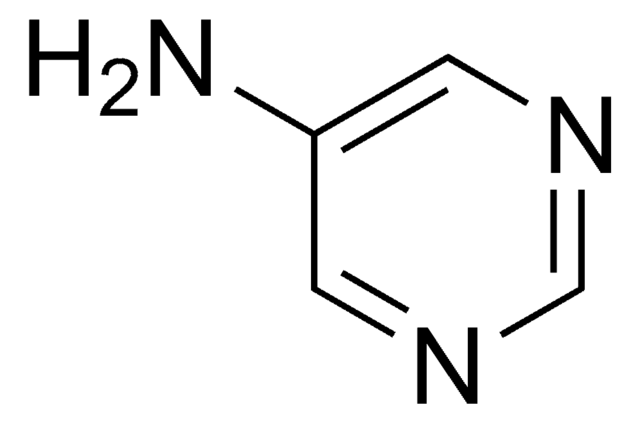
C4H5N3
CAS Number:
Molecular Weight:
95.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
154-156 °C (lit.)
SMILES string
Nc1ccncn1
InChI
1S/C4H5N3/c5-4-1-2-6-3-7-4/h1-3H,(H2,5,6,7)
InChI key
OYRRZWATULMEPF-UHFFFAOYSA-N
General description
The consequences of one-electron oxidation and one-electron reduction were studied for 4-aminopyrimidine.
Application
4-Aminopyrimidine has been used in the preparation of 1:4-dihydro-4-imino-1-methylpyrimidine hydriodide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dana Nachtigallová et al.
Journal of the American Chemical Society, 132(24), 8261-8263 (2010-06-02)
Nonadiabatic photodynamical simulations of 4-aminopyrimidine (4-APy) used as a model for adenine were performed by embedding it between two stacking methyl-guanine (mGua) molecules to determine the effect of spatial restrictions on the ultrafast photodeactivation mechanism of this nucleobase. A hybrid
Potential roles of the aminopyrimidine ring in thiamin catalyzed reactions.
F Jordan et al.
Annals of the New York Academy of Sciences, 378, 14-31 (1982-01-01)
R Friedemann et al.
Biochimica et biophysica acta, 1385(2), 245-250 (1998-07-10)
Ab initio calculations on the HF-SCF 6-31g* level were performed on tautomers as well as protonated and deprotonated species of thiamin. Aspects of the proton relay function of the 4'-aminopyrimidine ring in the thiamin catalysis were studied on model systems.
A Schellenberger
Biochimica et biophysica acta, 1385(2), 177-186 (1998-07-10)
The mechanism of ThDP enzymes originates in the anionic (ylid) structure of the coenzyme. On the other hand, no ylid species (as permanently existing structure) could be detected by 13C2-NMR studies with PDC (yeast), when the cofactor binds to the
Ewa D Raczyńska et al.
Journal of molecular modeling, 18(8), 3523-3533 (2012-02-14)
The consequences of one-electron oxidation and one-electron reduction were studied for 4-aminopyrimidine (4APM), which displays prototropic tautomerism. Since experimental techniques are incapable of detecting less than 0.1% of minor tautomers, quantum-chemical calculations [DFT(B3LYP)/6-311+G(d,p)] were carried out for all possible tautomers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service