247146
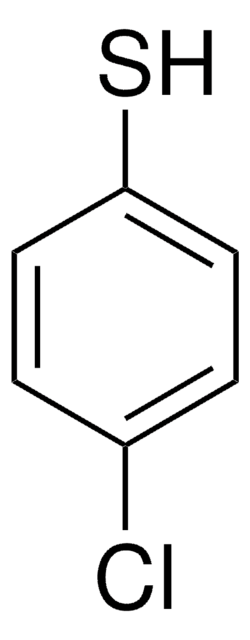
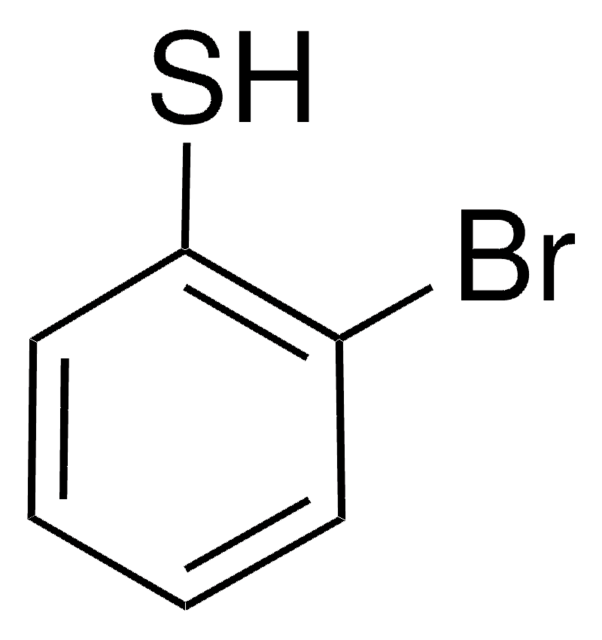
2-Chlorothiophenol
99%
Synonym(s):
2-Chlorobenzenethiol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4SH
CAS Number:
Molecular Weight:
144.62
Beilstein:
1209665
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.6029 (lit.)
bp
205-206 °C/260 mmHg (lit.)
density
1.275 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
Sc1ccccc1Cl
InChI
1S/C6H5ClS/c7-5-3-1-2-4-6(5)8/h1-4,8H
InChI key
PWOBDMNCYMQTCE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Chlorothiophenol undergoes multi-component condensation reaction with chloroacetyl chloride and primary aliphatic amines via Smile rearrrangement.
Application
2-Chlorothiophenol has been used in the synthesis of bis (2-chlorophenyl) analog of aromatic disulfide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Facile synthesis of 1, 4-benzothiazin-3-ones from Cu-catalyzed coupling of 2-iodoanilines and 2-mercaptoacetate.
Huang W-S, et al.
Tetrahedron Letters, 54(38), 5214-5216 (2013)
Bis (2-pyrimidinyl) disulfide dihydrate: a redetermination.
Jensen GB, et al.
Acta Crystallographica Section E, Structure Reports Online, 60(12), o2438-o2440 (2004)
Homan Kang et al.
Scientific reports, 5, 10144-10144 (2015-05-29)
Recently, preparation and screening of compound libraries remain one of the most challenging tasks in drug discovery, biomarker detection, and biomolecular profiling processes. So far, several distinct encoding/decoding methods such as chemical encoding, graphical encoding, and optical encoding have been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service