238732
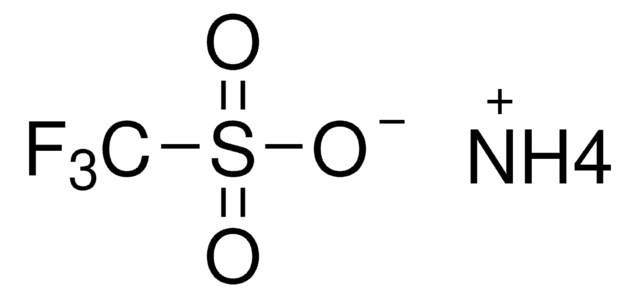
Ammonium trifluoroacetate
98%
Synonym(s):
Trifluoroacetic acid ammonium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3CO2NH4
CAS Number:
Molecular Weight:
131.05
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
123-125 °C (lit.)
functional group
carboxylic acid
fluoro
SMILES string
N.OC(=O)C(F)(F)F
InChI
1S/C2HF3O2.H3N/c3-2(4,5)1(6)7;/h(H,6,7);1H3
InChI key
YCNIBOIOWCTRCL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ammonium Trifluoroacetate is used as a catalyst in organic synthesis and as an additive in the mobile phase for chiral racemate separation.
Application
Used in the synthesis of hexafluoro-2-aminopentan-4-one ligand and in tuning and calibrating new liquid chromatography/mass spectrometry systems.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ammonium Trifluoroacetate-Mediated Synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones
Chandran R et al.
International Scholarly Research Network, 2011 (2011)
Garrett Hellinghausen et al.
Chirality, 30(9), 1067-1078 (2018-07-04)
A modified macrocyclic glycopeptide-based chiral stationary phase (CSP), prepared via Edman degradation of vancomycin, was evaluated as a chiral selector for the first time. Its applicability was compared with other macrocyclic glycopeptide-based CSPs: TeicoShell and VancoShell. In addition, another modified
Garrett Hellinghausen et al.
Journal of pharmaceutical and biomedical analysis, 155, 70-81 (2018-04-07)
Core-shell particles (superficially porous particles, SPPs) have been proven to provide high-throughput and effective separations of a variety of chiral molecules. However, due to their limited commercialization, many separations have not been reported with these stationary phases. In this study
Tejas Wattamwar et al.
European journal of mass spectrometry (Chichester, England), 26(2), 117-130 (2019-10-03)
A rapid and sensitive liquid chromatography-mass spectrometry method was developed, optimized, and validated for simultaneous quantification of empagliflozin and metformin in human plasma using empagliflozin D4and metformin D6 as an internal standard. Analytes and internal standard were extracted from plasma
Nimisha Thakur et al.
Chirality, 31(9), 688-699 (2019-07-19)
The enantiomeric excess of chiral starting materials is one of the important factors determining the enantiopurity of products in asymmetric synthesis. Fifty-one commercially available chiral reagents used as building blocks, catalysts, and auxiliaries in various enantioselective syntheses were assayed for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service