232777
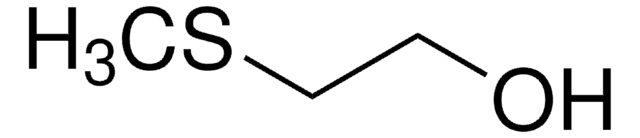
2-(Phenylthio)ethanol
99%
Synonym(s):
2-Hydroxyethyl phenyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SCH2CH2OH
CAS Number:
Molecular Weight:
154.23
Beilstein:
1860057
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.592 (lit.)
bp
115-116 °C/2 mmHg (lit.)
density
1.143 g/mL at 25 °C (lit.)
functional group
hydroxyl
thioether
SMILES string
OCCSc1ccccc1
InChI
1S/C8H10OS/c9-6-7-10-8-4-2-1-3-5-8/h1-5,9H,6-7H2
InChI key
KWWZHCSQVRVQGF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-(Phenylthio)ethanol has been used:
- in the synthesis of indole, benzofuran and benzothiophene
- for temporary masking of the thymine residue during the synthesis of sugar modified thymidine derivatives
- in the preparation of 4-[2-(phenylthio)ethoxy]phthalonitrile
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of indole, benzofuran and benzothiophene by reaction of 2-anilinoethanol, 2-phenoxyethanol and 2-(phenylthio) ethanol on AlPO4 and Pd/AlPO4 catalysts.
Afxantidis J, et al.
J. Mol. Catal. A: Chem., 102(1), 49-58 (1995)
2-(Phenylthio) ethyl as a novel two-stage base protecting group for thymidine analogues.
D'Onofrio J, et al.
Synlett, 2006(06), 845-848 null
Synthesis, characterization and electrochemistry of a new organosoluble metal-free and metallophthalocyanines.
Biyiklioglu Z, et al.
Polyhedron, 27(6), 1707-1713 (2008)
Xichang Dong et al.
Science (New York, N.Y.), 371(6528), 507-514 (2021-01-30)
Vicinal dibromides and dichlorides are important commodity chemicals and indispensable synthetic intermediates in modern chemistry that are traditionally synthesized using hazardous elemental chlorine and bromine. Meanwhile, the environmental persistence of halogenated pollutants necessitates improved approaches to accelerate their remediation. Here
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service