All Photos(2)
About This Item
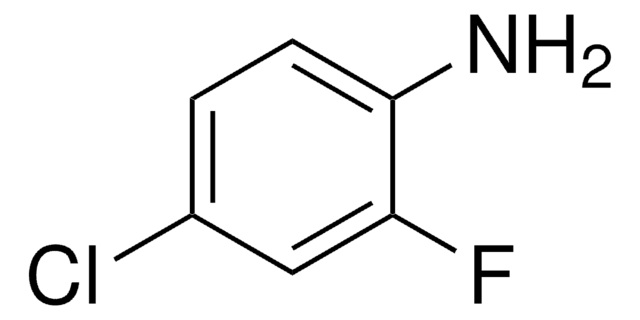
Linear Formula:
ClC6H3(F)NH2
CAS Number:
Molecular Weight:
145.56
Beilstein:
1562786
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
227-228 °C (lit.)
mp
42-44 °C (lit.)
functional group
chloro
fluoro
SMILES string
Nc1ccc(F)c(Cl)c1
InChI
1S/C6H5ClFN/c7-5-3-4(9)1-2-6(5)8/h1-3H,9H2
InChI key
YSEMCVGMNUUNRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The exposure of workers to 3-chloro-4-fluoroaniline (CFA) was monitored by an HPLC method.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P J Boogaard et al.
Environmental health perspectives, 102 Suppl 6, 23-25 (1994-10-01)
In two studies, involving 75 and 72 workers, potential exposure to 3-chloro-4-fluoroaniline (CFA) was biologically monitored by determination of its main urinary metabolite 2-amino-4-chloro-5-fluorophenol sulfate (CFA-S). As this method only allows the detection of recent exposure, analysis of CFA adducts
C V Eadsforth et al.
Journal of analytical toxicology, 12(6), 330-333 (1988-11-01)
An HPLC method for monitoring exposure of workers to 3-chloro-4-fluoroaniline (CFA) is described. It is based on the detection of a major urinary metabolite, 2-amino-4-chloro-5-fluorophenyl sulphate, and is superior to the previously adopted GC method. The limit of detection for
C J Duckett et al.
Xenobiotica; the fate of foreign compounds in biological systems, 36(1), 59-77 (2006-03-02)
The metabolic fate of 3-chloro-4-fluoroaniline was investigated in rat following intraperitoneal (i.p.) administration at 5 and 50 mg kg(-1) using a combination of HPLC-MS, HPLC-MS/MS, (19)F-NMR spectroscopy, HPLC-NMR spectroscopy and high-pressure liquid chromatography-inductively coupled plasma mass spectrometry (HPLC-ICPMS) with (35)Cl
M K Baldwin et al.
Xenobiotica; the fate of foreign compounds in biological systems, 10(2), 135-144 (1980-02-01)
1. 3-Chloro-4-fluorol[14C]aniline, orally administered to a female dog (0.135 mg/kg), was eliminated in the urine as 2-amino-4-chloro-5-fluorophenyl sulphate (83% in 48 h). 2. When 3-chloro-4-fluoro[14C]aniline was administered orally to male rats (ca 2.3 mg/kg), the 0-24 h urine contained 2-amino-4-chloro-5-fluorophenyl
Vasili Travkin et al.
FEMS microbiology letters, 209(2), 307-312 (2002-05-15)
In this paper we report the isolation and characterization of an anaerobic enrichment culture as well as of a Rhodococcus sp. strain 2 capable of degrading 3,4-dihaloanilines under nitrate reducing conditions. Using mass spectrometry several of the intermediates formed in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service