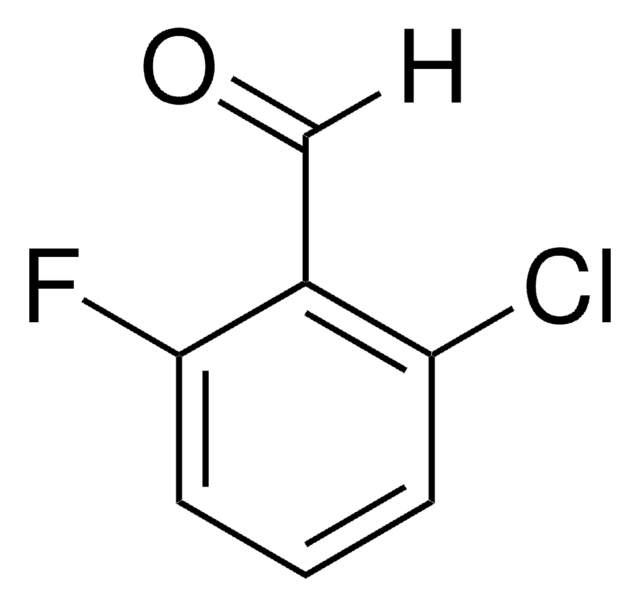
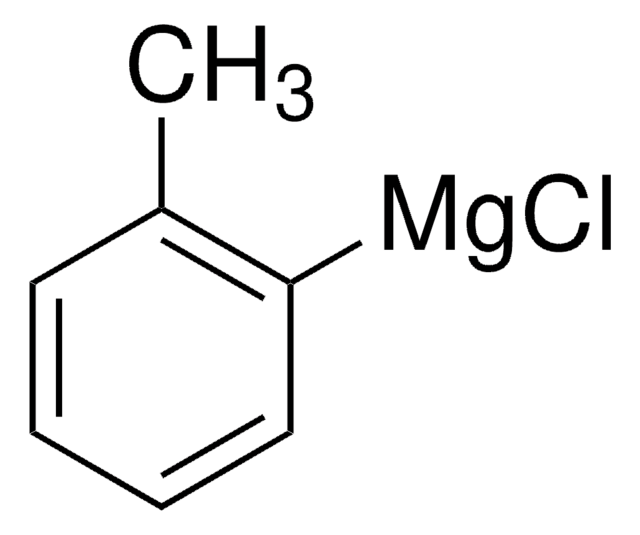
224448
Phenylmagnesium chloride solution
2.0 M in THF
Synonym(s):
Chlorophenylmagnesium
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5MgCl
CAS Number:
Molecular Weight:
136.86
Beilstein:
3587900
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
2.0 M in THF
density
~1.02 g/mL at 20 °C
1.042 g/mL at 25 °C
SMILES string
Cl[Mg]c1ccccc1
InChI
1S/C6H5.ClH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
GQONLASZRVFGHI-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Phenylmagnesium chloride (PhMgCl) solution is a common Grignard reagent used in the synthesis of (−)-phenserine and stephacidin B. It is employed in a variety of cross-coupling reactions. It can also be used as an electrolyte solution along with aluminium chloride (AlCl3) in the rechargeable magnesium batteries.
Packaging
Other Notes
Storage below 25°C may cause formation of crystalline magnesium salts. Moving container to a warm location and occasional gentle swirling should redissolve the solid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asymmetric synthesis of pyrrolidinoindolines. Application for the practical total synthesis of (−)-phenserine
Huang A, et al.
Journal of the American Chemical Society, 126(43), 14043-14053 (2004)
Enantioselective synthesis of stephacidin B.
Herzon S, et al.
Journal of the American Chemical Society, 127(15), 5342-5344 (2005)
Nickel-Catalyzed Cross-Coupling of Aryl Chlorides with Aryl Grignard Reagents.
B ohm VPW, et al.
Angewandte Chemie (International Edition in English), 39(9), 1602-1604 (2000)
Electrolyte solutions with a wide electrochemical window for rechargeable magnesium batteries.
Mizrahi O, et al.
Journal of the Electrochemical Society, 155(2), A103-A109 (2008)
Functional group tolerant Kumada- Corriu- Tamao coupling of nonactivated alkyl halides with aryl and heteroaryl nucleophiles: Catalysis by a nickel pincer complex permits the coupling of functionalized Grignard reagents.
Vechorkin O, et al.
Journal of the American Chemical Society, 131(28), 9756-9766 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service