All Photos(2)
About This Item
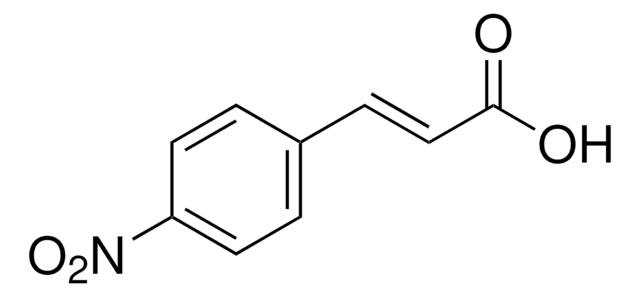
Linear Formula:
FC6H4CH=CHCO2H
CAS Number:
Molecular Weight:
166.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
209-210 °C (lit.)
SMILES string
OC(=O)\C=C\c1ccc(F)cc1
InChI
1S/C9H7FO2/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-6H,(H,11,12)/b6-3+
InChI key
ISMMYAZSUSYVQG-ZZXKWVIFSA-N
General description
The shock loading of 4-fluorocinnamic acid (4-FCA) was treated using a rotating biological contactor (RBC).
Application
4-Fluorocinnamic acid was used in growth medium for the Arthrobacter sp. strain G1.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Catarina L Amorim et al.
Bioresource technology, 144, 554-562 (2013-08-01)
A rotating biological contactor (RBC) was used to treat shock loadings of 4-fluorocinnamic acid (4-FCA). Intermittent 4-FCA shocks of 35 mg L(-1) were applied (ca. 3 months) with only limited mineralization occurring and accumulation of 4-fluorobenzoate (4-FBA) as an intermediate.
Syed A Hasan et al.
Biodegradation, 23(1), 117-125 (2011-07-06)
Arthrobacter sp. strain G1 is able to grow on 4-fluorocinnamic acid (4-FCA) as sole carbon source. The organism converts 4-FCA into 4-fluorobenzoic acid (4-FBA) and utilizes the two-carbon side-chain for growth with some formation of 4-fluoroacetophenone as a dead-end side
Ivana Perković et al.
European journal of medicinal chemistry, 187, 111927-111927 (2019-12-08)
Harmicines constitute novel hybrid compounds that combine two agents with reported antiplasmodial properties, namely β-carboline harmine and a cinnamic acid derivative (CAD). Cu(I) catalyzed azide-alkyne cycloaddition was employed for the preparation of three classes of hybrid molecules: N-harmicines 6a-i, O-harmicines
Marina Marinović et al.
Molecules (Basel, Switzerland), 25(19) (2020-09-27)
Harmicines represent hybrid compounds composed of β-carboline alkaloid harmine and cinnamic acid derivatives (CADs). In this paper we report the synthesis of amide-type harmicines and the evaluation of their biological activity. N-harmicines 5a-f and O-harmicines 6a-h were prepared by a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)


