220884
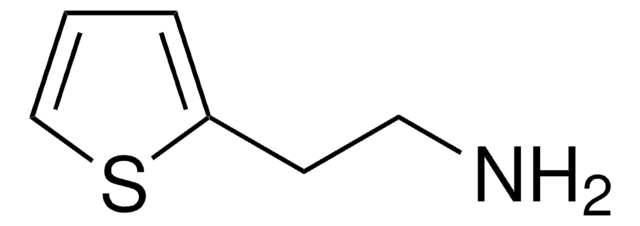
2-Thiophenemethylamine
96%
Synonym(s):
2-(Aminomethyl)thiophene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H7NS
CAS Number:
Molecular Weight:
113.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
liquid
refractive index
n20/D 1.5670 (lit.)
bp
95-99 °C/28 mmHg (lit.)
density
1.103 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
NCc1cccs1
InChI
1S/C5H7NS/c6-4-5-2-1-3-7-5/h1-3H,4,6H2
InChI key
FKKJJPMGAWGYPN-UHFFFAOYSA-N
General description
2-Thiophenemethylamine is a potential ligand replacement for poly(3-hexylthiophene)/CdSe hybrid solar cells.
Application
2-Thiophenemethylamine was used in preparation of:
- naphthalene-thiophene hybrid molecule (Z)-1-((thiophen-2-ylmethylamino)methylene)naphthalen-2(1H)-one
- fluorescent Pd2+ sensor, N-butyl-4-(p-methyloxy)-phenylethynyl-5-thiophenemethylamino-1,8-naphthalimide
Reactant involved in synthesis of:
Reactant involved in:
- Triazole-linked-thiopene conjugates for use as a biomimetic model for studies of metal detoxification and oxidative stress involving metallothionein
- Serotonin 5-HT1A receptor antagonists which have neuroprotective affects against ischemic cell damage
- Imidazole- and piperonyl-containing thiadiazoles and pyrimidines for use as inducible oxide synthase dimerization inhibitors
- Optoelectronic segmented polyurethanes
Reactant involved in:
- Studies of organocatalyzed asymmetric reductive amination of ketones
- Metal-free aerobic oxidative coupling of amines to imines
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Debasis Karak et al.
Dalton transactions (Cambridge, England : 2003), 42(19), 6708-6715 (2013-04-10)
A naphthalene-thiophene hybrid molecule (Z)-1-((thiophen-2-ylmethylamino)methylene)naphthalen-2(1H)-one () was prepared by condensation of 2-thiophenemethylamine and 2-hydroxy-1-naphthaldehyde. According to FTIR, (1)H NMR spectrometry and single crystal X-ray analysis, exists in the cis-keto-amine tautomeric form. behaves like a molecular AND type binary logic gate
Liping Duan et al.
Chemical communications (Cambridge, England), (47)(47), 6339-6341 (2008-12-03)
A new fluorescent Pd2+ sensor , N-butyl-4-(p-methyloxy)-phenylethynyl-5-thiophenemethylamino-1,8-naphthalimide, was designed and synthesized. It showed highly selective on-off fluorescence changes for Pd2+ among the representative transition and heavy metallic cations, and its fluorescence was efficiently quenched by 5 equivalents of Pd2+ in
Jun Yan Lek et al.
ACS applied materials & interfaces, 3(2), 287-292 (2011-01-26)
For hybrid solar cells, interfacial chemistry is one of the most critical factors for good device performance. We have demonstrated that the size of the surface ligands and the dispersion of nanoparticles in the solvent and in the polymer are
Adesola Abimbola Adeleke et al.
Journal of inorganic biochemistry, 214, 111266-111266 (2020-11-10)
Synthesis and spectroscopic characterization of five ligands ((E)-2-((pyridin-2-ylmethylene)amino)phenol L1, 2-(pyridin-2-yl)benzo[d]thiazole L2, (E)-N-(2-fluorophenyl)-1-(pyridin-2-yl)methanimine L3, (E)-1-(pyridin-2-yl)-N-(p-tolyl)methanimine L4 and (E)-1-(pyridin-2-yl)-N-(thiophen-2-ylmethyl)methanimine L5 along with fifteen silver(I) complexes of L1 - L5, with a general formula [AgL2]+X- (L = Schiff base and X = NO3-, ClO4- or CF3SO3-) is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service