20615
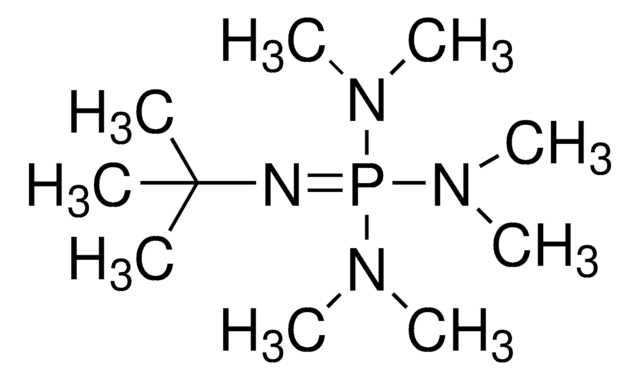
2-tert-Butyl-1,1,3,3-tetramethylguanidine
≥97.0% (GC)
Synonym(s):
BTMG
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H21N3
CAS Number:
Molecular Weight:
171.28
Beilstein:
2352408
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
form
liquid
refractive index
n20/D 1.457
bp
88-89 °C/43 mmHg (lit.)
functional group
amine
SMILES string
CN(C)\C(=N/C(C)(C)C)N(C)C
InChI
1S/C9H21N3/c1-9(2,3)10-8(11(4)5)12(6)7/h1-7H3
InChI key
YQHJFPFNGVDEDT-UHFFFAOYSA-N
General description
2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton′s base) is an excellent alternative to traditional inorganic bases for promoting the coupling reaction.
Application
- Synthesis of dinaphthyl ethers: Barton′s base, which includes 2-tert-Butyl-1,1,3,3-tetramethylguanidine, was utilized to promote SNAr reactions for the synthesis of highly oxygenated dinaphthyl ethers, demonstrating its efficacy as a catalyst in complex organic synthesis processes (Wipf and Lynch, 2003).
Caution
Remark on appearance: Material may form precipitate on storage. The precipitate may easily be separated by filtration.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
149.0 °F - closed cup
Flash Point(C)
65.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D.H.R. Barton et al.
Organic Syntheses, 74, 103-103 (1997)
Peter Wipf et al.
Organic letters, 5(7), 1155-1158 (2003-03-28)
[reaction: see text] Electron-rich dinaphthyl ethers were synthesized by S(N)Ar reactions between naphthols and activated fluoronaphthalenes. 2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton's base) was found to be an excellent, mild alternative to traditional inorganic bases for promoting the coupling reaction.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)




![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)

![1,5-Diazabicyclo[4.3.0]non-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/400/401/859b2474-712b-4448-b231-74d0bc3203f1/640/859b2474-712b-4448-b231-74d0bc3203f1.png)