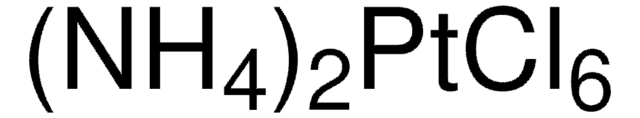206113
Platinum(IV) chloride
96%
Synonym(s):
Platinum tetrachloride
About This Item
Recommended Products
Quality Level
Assay
96%
form
powder and chunks
reaction suitability
reagent type: catalyst
core: platinum
mp
370 °C (dec.) (lit.)
density
4.303 g/mL at 25 °C (lit.)
SMILES string
Cl[Pt](Cl)(Cl)Cl
InChI
1S/4ClH.Pt/h4*1H;/q;;;;+4/p-4
InChI key
FBEIPJNQGITEBL-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Application
Due to its high affinity to the glycosyl acceptor hydroxy groups and bidentate ligation capacity, it can be used as a dual catalyst for regio- and stereoselective glycosidation reactions.
It can also be used for the photosensitization and surface modification of TiO2 for the photodegradation of 4-chlorophenol.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service