196525
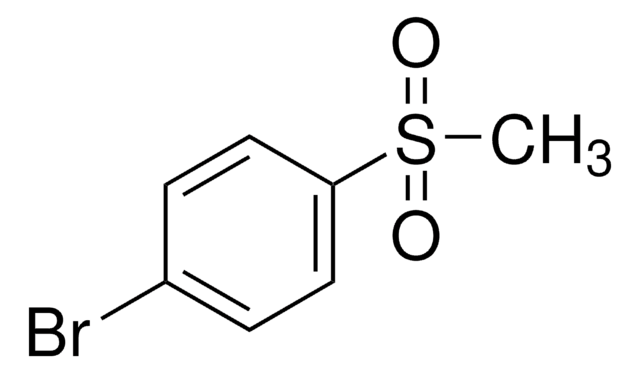
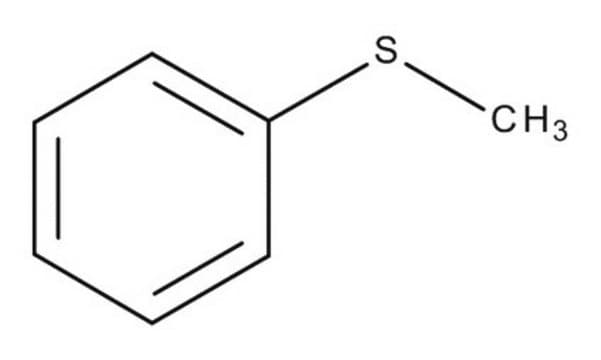
4-Bromothioanisole
97%
Synonym(s):
4-Bromophenyl methyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H4SCH3
CAS Number:
Molecular Weight:
203.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
128-130 °C/10 mmHg (lit.)
mp
38-40 °C (lit.)
functional group
bromo
thioether
SMILES string
CSc1ccc(Br)cc1
InChI
1S/C7H7BrS/c1-9-7-4-2-6(8)3-5-7/h2-5H,1H3
InChI key
YEUYZNNBXLMFCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Bromothioanisole undergoes Heck olefination reaction with styrenes to yield stilbenes.
Application
4-Bromothioanisole was used in the synthesis of:
- 4′-nitro-4-mercaptobiphenyl
- 4′-iodo-4-mercaptobiphenyl
- 3′-nitro-4-mercaptobiphenyl
- 3′-iodo-4-mercaptiobiphenyl
- (S)-(–)-p-bromophenyl methyl sulfoxide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective Oxidation of an Alkyl Aryl Sulfide: Synthesis of (S)-(-)-Methyl P-Bromophenyl Sulfoxide.
Drago C, et al.
Organic Syntheses, 86, 121-129 (2009)
Novel 1, 2-diarylcyclobutenes: Selective and orally active COX-2 inhibitors.
Friesen RW, et al.
Bioorganic & Medicinal Chemistry Letters, 6(22), 2677-2682 (1996)
Rupa Hiremath et al.
Chemical communications (Cambridge, England), (23)(23), 2676-2677 (2004-11-30)
Orthorhombic and triclinic crystals of 2-iodo-4-nitroaniline (INA) grow concomitantly from supersaturated ethanol solutions, but the less stable orthorhombic phase can be selectively grown on 3'-X-4-mercaptobiphenyl (X = NO(2), I) self-assembled monolayer templates.
Vasiliy Yu Evtushok et al.
Frontiers in chemistry, 7, 858-858 (2020-01-11)
In this work, we elaborated heterogeneous catalysts on the basis of the Venturello complex [PO4{WO(O2)2}4]3- (PW4) and nitrogen-free or nitrogen-doped carbon nanotubes (CNTs or N-CNTs) for epoxidation of alkenes and sulfoxidation of thioethers with aqueous hydrogen peroxide. Catalysts PW4/CNTs and
Isolation of S-(bromophenyl)cysteine isomers from liver proteins of bromobenzene-treated rats.
P E Weller et al.
Chemical research in toxicology, 4(1), 17-20 (1991-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service