195596
sec-Butyllithium solution
1.4 M in cyclohexane
Synonym(s):
Lithium-2-butanide, s-BuLi
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
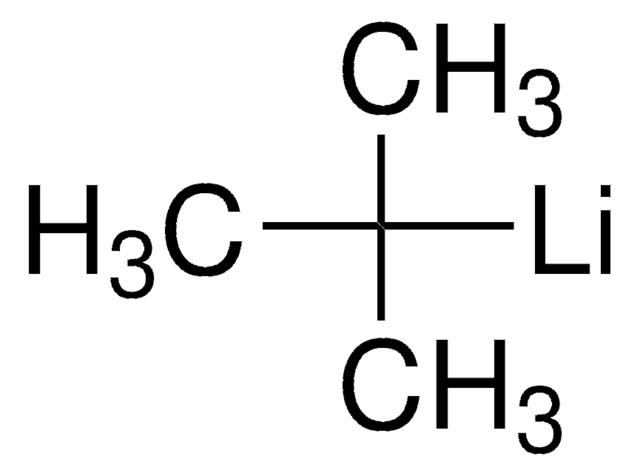
CH3CH2CH(CH3)Li
CAS Number:
Molecular Weight:
64.06
Beilstein:
3587206
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
concentration
1.4 M in cyclohexane
density
0.769 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[Li]C(C)CC
InChI
1S/C4H9.Li/c1-3-4-2;/h3H,4H2,1-2H3;
InChI key
VATDYQWILMGLEW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
sec-Butyllithium solution (1.4M in cyclohexane) has been used in the multi-step synthesis of 5-methyl-5,6-dihydrothymidine (5-MDHT) from thymidine. It has also been used in the copper-catalyzed asymmetric allylic alkylation (AAA) of allyl bromides, chlorides, and ethers in the presence of a chiral ligand.
Packaging
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
Legal Information
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cheng Chen et al.
Organic & biomolecular chemistry, 15(25), 5364-5372 (2017-06-16)
A series of 6,6-dihalo-2-azabicyclo[3.1.0]hexane and 7,7-dihalo-2-azabicyclo[4.1.0]heptane compounds were prepared by the reaction of dihalocarbene species with N-Boc-2,3-dihydro-1H-pyrroles or -1,2,3,4-tetrahydropyridines. Monochloro substrates were synthesised as well, using a chlorine-to-lithium exchange reaction. The behaviour of several aldehydes and ketones under reductive amination
Dimitrios Moschovas et al.
Nanomaterials (Basel, Switzerland), 10(8) (2020-08-06)
The synthesis, molecular and morphological characterization of a 3-miktoarm star terpolymer of polystyrene (PS, M¯n = 61.0 kg/mol), polybutadiene (PB, M¯n = 38.2 kg/mol) and polyisoprene (PI, M¯n = 29.2 kg/mol), corresponding to volume fractions (φ) of 0.46, 0.31 and
Jason V Chari et al.
The Journal of organic chemistry, 84(6), 3652-3655 (2019-03-07)
Silyl triflate precursors to cyclic alkynes and allenes serve as valuable synthetic building blocks. We report a concise and scalable synthetic approach to prepare the silyl triflate precursors to cyclohexyne and 1,2-cyclohexadiene. The strategy involves a retro-Brook rearrangement of an
Lukasz Otulakowski et al.
Polymers, 12(1) (2020-01-17)
In this work, the self-assembly of a series of amphiphilic polystyrene-b-polyglycidol (PS-b-PGL) diblock copolymers in dioxane and dioxane/water mixtures is presented. The PS-b-PGL have an average degree of polymerization (DP) of PS block equal to 29 units and varied degrees
Leixing Chen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(68), 18012-18019 (2018-11-15)
Although living polymerization methods are widely applicable to organic monomers, their application to inorganic monomers is rare. For the first time, we show that the living poly(methylenephosphine) (PMPn- ) anion can function as a macroinitiator for olefins. Specifically, the phosphaalkene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service