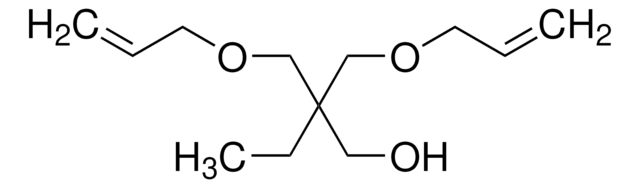
191523
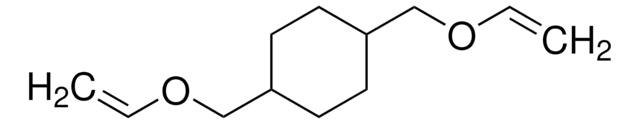
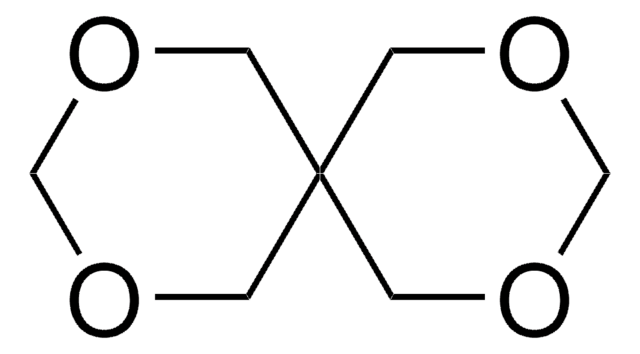
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H16O4
CAS Number:
Molecular Weight:
212.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
108-110 °C/2 mmHg (lit.)
mp
43-46 °C (lit.)
density
1.251 g/mL at 25 °C (lit.)
SMILES string
C=CC1OCC2(CO1)COC(OC2)C=C
InChI
1S/C11H16O4/c1-3-9-12-5-11(6-13-9)7-14-10(4-2)15-8-11/h3-4,9-10H,1-2,5-8H2
InChI key
OOXMQACSWCZQLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane is an acetal-type crosslinking agent comonomer and has been used:
- in radical emulsion copolymerization of 2-hydroxyethyl methacrylate
- as cross-linking agent in the synthesis of acid-degradable core-crosslinked micelles
- in the synthesis of new biocompatible copolymer for loading the indomethacin as drug model
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Upon the emulsion polymerization of 2-hydroxyethyl methacrylate with 3, 9-divinyl-2, 4, 8, 10-tetraoxaspiro [5.5]-undecane.
Nita LE, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 381(1), 111-117 (2011)
S Stern et al.
Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology, 41(2), 143-149 (1996-11-01)
Solid tumours contain hypoxic cells which are resistant to radiotherapy. This study compares the efficacy of several strategies to counteract diffusion-limited hypoxia, or intermittent hypoxia in a fractionated regimen of 1 to 6 x 2 Gy. Nicotinamide (250 mg/kg), perflubron
Acid-Degradable Core-Crosslinked Micelles Prepared from Thermosensitive Glycopolymers Synthesized via RAFT Polymerization.
Zhang L, et al.
Macromolecular Rapid Communications, 29(2), 123-129 (2008)
Loredana E Nita et al.
Journal of materials science. Materials in medicine, 23(5), 1211-1223 (2012-03-15)
The study presents the possibility to use the 2-hydroxyethyl methacrylate--HEMA copolymer with a comonomer with spiroacetal moiety, 3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5]-undecane)-U, as polymer network for loading the indomethacin--INN as drug model, and also, the controlled release evaluation of the prepared bioactive system. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service