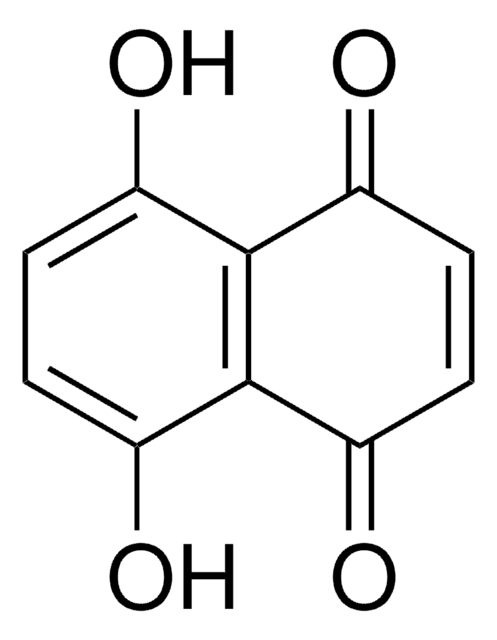
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H8O3
CAS Number:
Molecular Weight:
188.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
184-187 °C (lit.)
functional group
ether
ketone
SMILES string
COC1=CC(=O)c2ccccc2C1=O
InChI
1S/C11H8O3/c1-14-10-6-9(12)7-4-2-3-5-8(7)11(10)13/h2-6H,1H3
InChI key
OBGBGHKYJAOXRR-UHFFFAOYSA-N
Related Categories
General description
2-Methoxy-1,4-naphthoquinone is a potential candidate for Helicobacter pylori infection related disease therapy. It is isolated from the leaves of Impatiens glandulifera.
Application
2-Methoxy-1,4-naphthoquinone was used in the preparation of 2-(4-X-phenylene)amine-1,4-naphthoquinones (X= ferrocenyl, OMe, Me, I, Cl and NO2).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Ishiguro et al.
Journal of natural products, 61(9), 1126-1129 (1998-09-28)
Dinaphthofuran-7,12-dione derivatives named balsaminones A (1) and B (2) were isolated from the pericarp of Impatiens balsamina L. together with the known compound 2-methoxy-1,4-naphthoquinone (3). Their structures were elucidated by spectral techniques. These compounds have significant antipruritic activity.
General method for the high yield preparation of 2-(4-X-phenylene) amine-1, 4-naphthoquinones (X= ferrocenyl, OMe, Me, I, Cl, and NO2) from 2-methoxy-1, 4-naphthoquinone and investigation of H+ and Mg2+ catalysts with DFT calculations.
Francisco AI, et al.
Journal of Molecular Structure, 891(1), 228-232 (2008)
Aldana L Zalazar et al.
Food research international (Ottawa, Ont.), 116, 916-924 (2019-02-06)
Probabilistic microbial modelling using logistic regression was used to predict the growth/no growth (G/NG) interfaces of Zygosaccharomyces bailii in simulated acid sauces as a function of natamycin, xanthan gum (XG) and sodium chloride concentrations. The growth was assessed colorimetrically by
Scott B Vafai et al.
PloS one, 11(9), e0162686-e0162686 (2016-09-14)
Deficiency of mitochondrial complex I is encountered in both rare and common diseases, but we have limited therapeutic options to treat this lesion to the oxidative phosphorylation system (OXPHOS). Idebenone and menadione are redox-active molecules capable of rescuing OXPHOS activity
Naomi Mori et al.
Journal of natural medicines, 65(1), 234-236 (2010-10-05)
A screening study using a luciferase assay to identify natural products which inhibit Wnt signaling was carried out. The bioassay-guided fractionation of aerial parts of a plant, Impatiens balsamina, led to the isolation of 2-methoxy-1,4-naphthoquinone (1) as an active compound.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service