178470
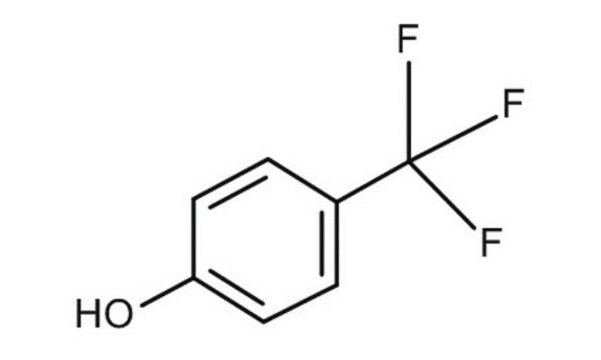
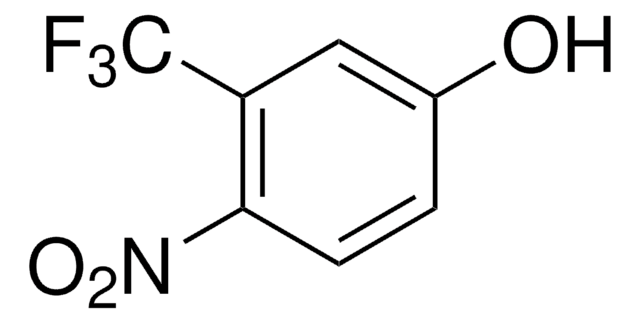
4-(Trifluoromethyl)phenol
97%
Synonym(s):
α,α,α-Trifluoro-p-cresol, 4-Hydroxybenzotrifluoride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CF3C6H4OH
CAS Number:
Molecular Weight:
162.11
Beilstein:
1637019
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
mp
45-47 °C (lit.)
functional group
fluoro
storage temp.
2-8°C
SMILES string
Oc1ccc(cc1)C(F)(F)F
InChI
1S/C7H5F3O/c8-7(9,10)5-1-3-6(11)4-2-5/h1-4,11H
InChI key
BAYGVMXZJBFEMB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-(Trifluoromethyl)phenol molecule, bound at the active site of H61T (His-61→Thr) mutant, shows strong density.
4-(Trifluoromethyl)phenol also known as p-trifluoromethylphenol, is used in synthesis of polymers and monomers.
4-(Trifluoromethyl)phenol also known as p-trifluoromethylphenol, is used in synthesis of polymers and monomers.
Application
4-(Trifluoromethyl)phenol (4-hydroxybenzotrifluoride) was used in the synthesis of diaryl ether.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jin-Kyun Lee et al.
Chemical communications (Cambridge, England), (39)(39), 4780-4782 (2008-10-03)
A high yielding, batch mode synthesis of diaryl ethers and sulfides by an S(N)Ar fluoride-mediated process in scCO(2) has been developed; the use of a polymer-supported imidazolium fluoride reagent in batch mode led to the development of a fixed-bed continuous
B van de Wier et al.
Chemico-biological interactions, 242, 139-144 (2015-10-03)
Cytochrome P450 2E1 (CYP2E1) expression and activity in the liver is associated with the degree of liver damage in patients with alcoholic steatohepatitis (ASH) as well as non-alcoholic steatohepatitis (NASH). CYP2E1 is known to generate reactive oxygen species, which leads
Selina Tisler et al.
Environmental science & technology, 53(13), 7400-7409 (2019-05-29)
The present study investigates the transformation of the antidepressant fluoxetine (FLX) by photo- and biodegradation and shows similarities and differences in transformation products (TPs). TPs were identified using LC-high-resolution mass spectrometry with positive and negative electrospray ionization. In a sunlight
D C Thompson et al.
Chemico-biological interactions, 126(1), 1-14 (2000-05-29)
4-Trifluoromethylphenol (4-TFMP) was cytotoxic to precision-cut rat liver slices as indicated by loss of intracellular potassium. Intracellular glutathione levels decreased and fluoride ion levels increased in a time and concentration-dependent manner. The cytotoxicity of 4-TFMP did not appear to be
Synthesis of Poly-p-oxyperfluorobenzylene and Related Polymers. A Novel Synthesis of the Monomer 2, 3, 5, 6-Tetrafluoro-4-trifluoromethylphenol
Journal of Research of the National Bureau of Standards, 71, 33-33 (1967)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service