All Photos(1)
About This Item
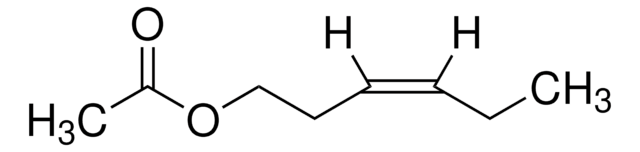
Linear Formula:
CH3COO(CH2)5CH3
CAS Number:
Molecular Weight:
144.21
Beilstein:
1747138
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.409 (lit.)
bp
168-170 °C (lit.)
mp
−80 °C (lit.)
density
0.87 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCCCCCOC(C)=O
InChI
1S/C8H16O2/c1-3-4-5-6-7-10-8(2)9/h3-7H2,1-2H3
InChI key
AOGQPLXWSUTHQB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hexyl acetate is an ester and is commonly used as a solvent for resins, polymers, fats, and oils. It can also be used as a flavoring agent in the food industry. It is produced by acid catalyzed liquid phase esterification of n-hexanol and acetic acid.
Application
Hexyl acetate was used to study the activity of diamondback moth sex pheromone and larval frass volatiles, as well as green leaf volatiles from cabbage, on the natural enemies of the pest.
Biochem/physiol Actions
Hexyl acetate has antimicrobial activity and can be used to improve the safety of minimally processed fruits. Hexyl acetate is a fruity smelling fluid used as flavoring agent or in perfumes. Hexyl acetate is a green leaf volatile from cabbage Brassica oleracea var. capitata L.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
132.8 °F - closed cup
Flash Point(C)
56 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Intensification of enzyme catalysed synthesis of hexyl acetate using sonication
AR Deshmukh, et al.
Green Processing and Synthesis, 6, 55-62 (2017)
G V P Reddy et al.
Journal of chemical ecology, 28(1), 131-143 (2002-03-02)
The parasitoids Trichogramma chilonis (Hymenoptera: Trichogrammatidae) and Cotesia plutellae (Hymenoptera: Braconidae), and the predator Chrysoperla carnea (Neuroptera: Chrysopidae), are potential biological control agents for the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). We present studies on the interactions between these bioagents
Application of hexanal, E-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples.
Rosalba Lanciotti et al.
Journal of agricultural and food chemistry, 51(10), 2958-2963 (2003-05-02)
The aims of this work were to evaluate the effects of different concentrations of hexanal, (E)-2-hexenal, hexyl acetate, and their mixtures on the fate of pathogenic species such as Escherichia coli, Salmonella enteritidis, and Listeria monocytogenes inoculated in model systems
Synthesis of n-hexyl acetate by reactive distillation.
Schmitt M, et al.
Chemical Engineering and Processing, 43(3), 397-409 (2004)
Valentina Canuti et al.
Journal of agricultural and food chemistry, 67(9), 2647-2659 (2019-02-14)
Sangiovese is the most widespread Italian red cultivar and constitutes the basis of internationally known wines such as Chianti and Brunello di Montalcino. Outside of Europe, Argentina is the largest producer, followed by the United States. This study sought to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service