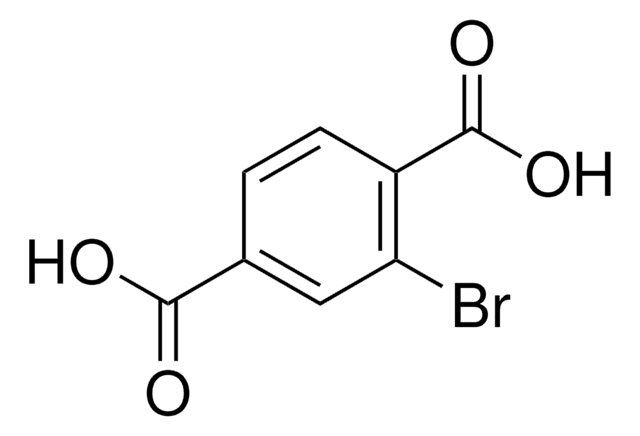
All Photos(1)
About This Item
Linear Formula:
I2C6H3CO2H
CAS Number:
Molecular Weight:
373.91
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
183-187 °C (lit.)
functional group
carboxylic acid
iodo
SMILES string
OC(=O)c1cc(I)ccc1I
InChI
1S/C7H4I2O2/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3H,(H,10,11)
InChI key
NSKPFWAAYDFCFS-UHFFFAOYSA-N
Application
2,5-Diiodobenzoic acid was used for detection and responses of divergent compounds which are strong electron absorbers by gas chromatography with electron capture detection.
Biochem/physiol Actions
2,5-Diiodobenzoic acid forms 1:1 complex with cycloheptaamylose. It undergoes palladium-catalyzed coupling reaction with terminal alkynes.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Highly regioselective palladium-catalyzed condensation of terminal acetylenes with 2, 5-diiodobenzoic acid.
Balavoine F, et al.
Tetrahedron Letters, 40(48), 8351-8354 (1999)
Attogram-level detection and relative sensitivity of strong electrophores by gas chromatography with electron capture detection.
Corkill JA, et al
Analytical Chemistry, 54(3), 481-485 (1982)
The crystal structure of a complex of cycloheptaamylose with 2,5-diiodobenzoic acid.
J A Hamilton et al.
Biochemical and biophysical research communications, 73(3), 659-664 (1976-12-06)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service