All Photos(1)
About This Item
Empirical Formula (Hill Notation):
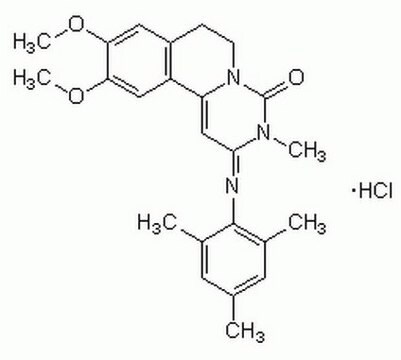
C24H27N3O3 · HCl
CAS Number:
Molecular Weight:
441.95
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98%
form
powder
SMILES string
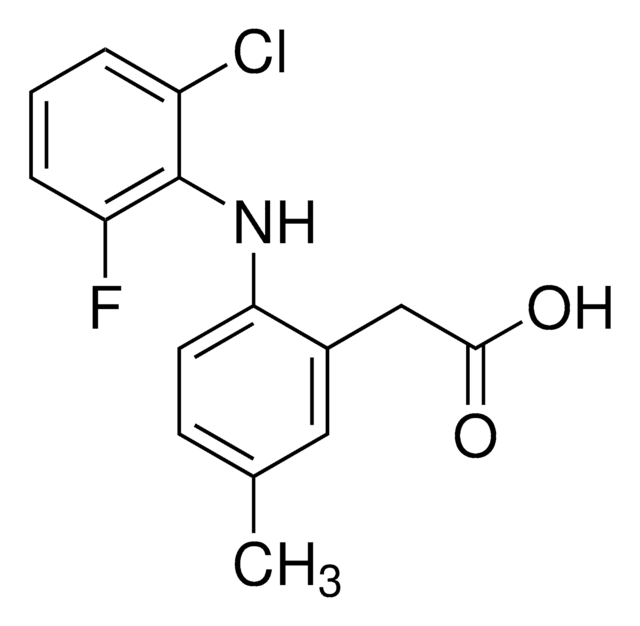
Cl[H].COc1cc2CCN3C(=O)N(C)C(\C=C3c2cc1OC)=N\c4c(C)cc(C)cc4C
InChI
1S/C24H27N3O3.ClH/c1-14-9-15(2)23(16(3)10-14)25-22-13-19-18-12-21(30-6)20(29-5)11-17(18)7-8-27(19)24(28)26(22)4;/h9-13H,7-8H2,1-6H3;1H/b25-22+;
InChI key
DTCZZBVPTHVXFA-OSMRDGEFSA-N
Gene Information
human ... PDE3A(5139) , PDE3B(5140)
Application
Trequinsin has been used as a PDE3 inhibitor in rat juxtaglomerular cells. This study reported that trequinsin can enhance cellular cAMP content, forskolin-induced cAMP synthesis, and renin release in cells.
Biochem/physiol Actions
Phosphodiesterase III inhibitor.
Trequinsin is a strong antihypertensive agent that has a hemodynamic profile similar to that of arteriolar dilators. Trequinsin can block platelet aggregation and also inhibit tissue factor expression in human endothelial cells,.
Features and Benefits
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Phosphodiesterases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Kurjak et al.
The American journal of physiology, 276(6 Pt 1), G1521-G1530 (1999-06-11)
The effect of nitric oxide (NO) on the release of bombesin-like immunoreactivity (BLI) was examined in synaptosomes of rat small intestine. The NO donor S-nitroso-N-acetylpenicillamine (SNAP; 10(-7) to 10(-4) M) significantly stimulated BLI release. In the presence of the NO
B Lal et al.
Journal of medicinal chemistry, 27(11), 1470-1480 (1984-11-01)
Series of 3-substituted-9,10-dimethoxy-3,4,6,7-tetrahydro-2H-pyrimido [6,1-a]isoquinoline-2,4-diones and 2-substituted-9,10-dimethoxy-6,7-dihydro-4H-pyrimido [6,1-a]isoquinolin-4-ones were synthesized and tested for blood pressure lowering properties in anesthetized normotensive cats and conscious spontaneously hypertensive rats. Several compounds in the 2-(arylamino)-9,10-dimethoxy-6,7-dihydro-4H-pyrimido [6,1-a]isoquinolin-4-one series display a high order of activity. The most
Ulla G Friis et al.
Circulation research, 90(9), 996-1003 (2002-05-23)
We tested the hypothesis that cGMP stimulates renin release through inhibition of the cAMP-specific phosphodiesterase 3 (PDE3) in isolated rat juxtaglomerular (JG) cells. In addition, we assessed the involvement of PDE4 in JG-cell function. JG cells expressed PDE3A and PDE3B
R F Booth et al.
Biochemical pharmacology, 36(20), 3517-3521 (1987-10-15)
The discovery and structure-activity of a new class of renal artery phosphodiesterase inhibitors is reported, some of which are highly selective for the guanosine cyclic 3',5'-monophosphate phosphodiesterase. One of these compounds, 5,6-dihydro-8,9,11,12-tetramethoxy-1,3-dioxo-1H-benz[f]- isoquino [8,1,2- hij]quinazoline-2(3H)-carboxylic acid, ethyl ester (9), is
R A Rius et al.
Life sciences, 54(22), 1735-1743 (1994-01-01)
In primary cultured bovine adrenal chromaffin cells (BACC), pituitary adenylate cyclase activating polypeptide 1-38 (PACAP) produced a dose related increase in tyrosine hydroxylase (TH) Vmax when measured 48 hours after the beginning of the treatment; a significant increase was observed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service