S1951
α-2,3-Sialyltransferase from Pasteurella multocida
recombinant, expressed in E. coli BL21, ≥2 units/mg protein
Synonym(s):
CMP-N-acetylneuraminate:β-D-galactoside α-(2,3)-N-acetylneuraminyltransferase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized powder
specific activity
≥2 units/mg protein
mol wt
46.4 kDa
shipped in
dry ice
storage temp.
−20°C
General description
Sialyltransferases belongs to the glycosyltransferases family with the capability of catalyzing the transfer of N-acetylneuraminic acid residues. It is a multifunctional enzyme with α-2,3-sialyltransferase activity, α-2,6-sialyltransferase activity, sialidase activity, and trans-sialidase activity. It has a molar mass of 46.4 kDa, with pI, pH being 5.94 and 7.5-8.5 respectively.
Biochem/physiol Actions
Sialyltransferase catalyzes the transfer of Neu5Ac from CMP-Neu5Ac as a donor substrate to the β-D-galactosyl-1,4-N-acetyl-D-glucosaminyl termini of acceptor molecules including glycoproteins, glycolipids, and oligosaccharides.
Sialyltransferase transfers Neu5Ac from CMP-Neu5Ac to the galactosyl terminus of acceptor molecules including glycoproteins, glycolipids, and oligosaccharides.
Unit Definition
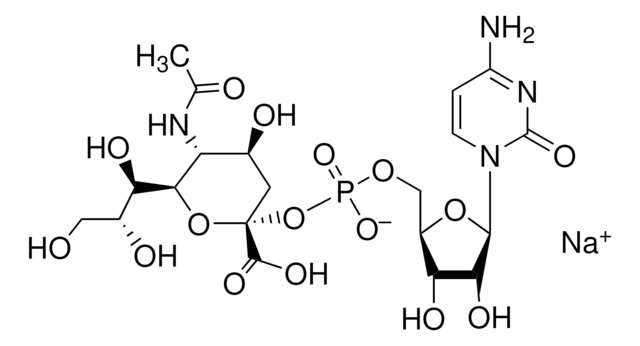
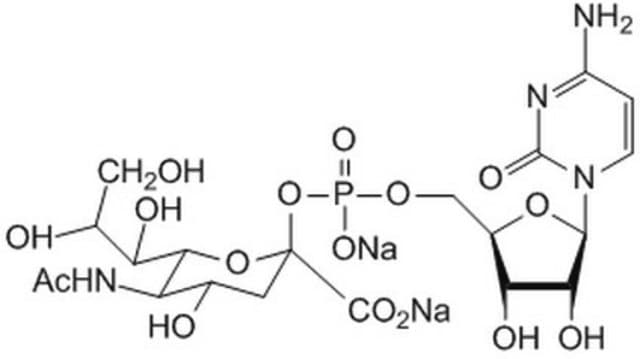
One unit will catalyze the formation of 1.0 μmol Neu-5-Ac-α-2,3LacMU from CMP-Neu-5-Ac and Lac-β−OMU per minute at 37 °C at pH 8.0.
Physical form
Lyophilized powder containing Tris-HCl and NaCl
Preparation Note
Reconstitute the lyophilized powder with a volume of water in the range of 0.1 mL to 1 mL, to give a concentration in the range of 1 unit/mL (1 mL volume of water) to 10 units/mL (0.1 mL volume of water).
Analysis Note
Enzymatic activity assays performed in Tris-HCl buffer (100 mM, pH 8.0) containing CMP-Neu-5Ac (1 mM) and Lac-β−OMU (1 mM) at 37°C for 30 min and analyzed using HPLC with a fluorescence detector (excitation at 325 nm and emission at 372 nm).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vireak Thon et al.
Applied microbiology and biotechnology, 94(4), 977-985 (2011-11-15)
Pasteurella multocida (Pm) strain Pm70 has three putative sialyltransferase genes including Pm0188, Pm0508, and Pm1174. A Pm0188 gene homolog in Pm strain P-1059 encodes a multifunctional α2-3-sialyltransferase, PmST1, that prefers oligosaccharide acceptors. A Pm0508 gene homolog in the same strain
Sung-Wook Son et al.
Biochemical and biophysical research communications, 414(1), 159-164 (2011-09-29)
In this study we investigated for the first time the transcriptional regulation of pig Galβ1,3GalNAc α2,3-sialyltransferase (pST3Gal I) in response to TGF-β1 in porcine kidney PK-15 cells. The pST3Gal I gene was found to span about 90kb and to be
Yoshimitsu Kakuta et al.
Glycobiology, 18(1), 66-73 (2007-10-27)
Sialyltransferases are a family of glycosyltransferases that catalyze the transfer of N-acetylneuraminic acid residues from cytidine monophosphate N-acetylneuraminic acid (CMP-NeuAc) as a donor substrate to the carbohydrate groups of glycoproteins and glycolipids as acceptor substrates. We determined the crystal structure
Lisheng Ni et al.
Biochemistry, 45(7), 2139-2148 (2006-02-16)
Sialyltransferases catalyze reactions that transfer a sialic acid from CMP-sialic acid to an acceptor (a structure terminated with galactose, N-acetylgalactosamine, or sialic acid). They are key enzymes that catalyze the synthesis of sialic acid-containing oligosaccharides, polysaccharides, and glycoconjugates that play
Vishwanath B Chachadi et al.
The international journal of biochemistry & cell biology, 43(4), 586-593 (2010-12-21)
Sialyl Lewis X is a tumor-associated antigen frequently found in the advanced cancers. However, the mechanism for the production of this cancer antigen is not entirely clear. The objective of this study is to examine whether epigenetics is involved in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service