M8515
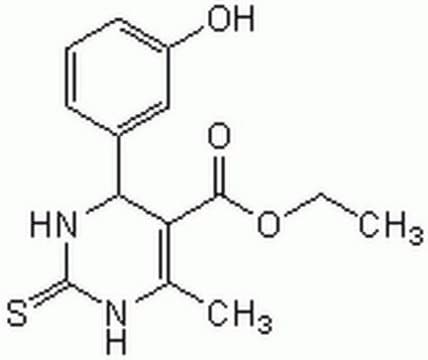
Monastrol
≥98% (HPLC), solid
Synonym(s):
4-(3-Hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydro-4H-pyrimidin-5-carboxylic Acid Ethyl Ester
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
storage condition
protect from light
color
white to off-white
mp
185-185.9 °C (lit.)
solubility
DMSO: >5 mg/mL
storage temp.
2-8°C
SMILES string
CCOC(=O)C1=C(C)NC(=S)NC1c2cccc(O)c2
InChI
1S/C14H16N2O3S/c1-3-19-13(18)11-8(2)15-14(20)16-12(11)9-5-4-6-10(17)7-9/h4-7,12,17H,3H2,1-2H3,(H2,15,16,20)
InChI key
LOBCDGHHHHGHFA-UHFFFAOYSA-N
Application
- to treat MDA-MB-231 cells as a non-microtubule-targeting agent
- as a antineoplastic agent, to treat mouse myeloma cell line SP 2/0, to induce apoptosis and to elucidate the role of metabotropic glutamate receptor 3 (Grm3) in apoptosis
- as an inhibitor of pteridine reductase in GFP-transfected promastigotes infected macrophages for flow cytometer-based growth inhibition assay and to evaluate anti-leishmanial activity of Leishmania donovani hamster model in vivo
Biochem/physiol Actions
Features and Benefits
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service