O3750
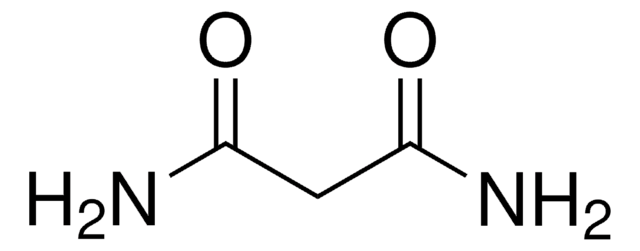
Oxamic acid
≥98%
Synonym(s):
Aminooxoacetic acid, Oxalic acid monoamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2COCO2H
CAS Number:
Molecular Weight:
89.05
Beilstein:
1743294
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder
mp
207-210 °C (dec.) (lit.)
SMILES string
NC(=O)C(O)=O
InChI
1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6)
InChI key
SOWBFZRMHSNYGE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Oxamic acid (OA) can be used as a reactant to prepare 6-phenanthridinecarboxamide by direct C-H carbamoylation reaction using ammonium persulfate in DMSO. It can also be used as an organic ligand to prepare functionalized metal oxide nanoparticles for various biological applications. OA along with p-aminobenzoic acid is used to functionalize Au nanoparticles for the development of a sensor to detect Fe3+ ions by the calorimetric method.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adam D Moorhouse et al.
Chemical communications (Cambridge, England), 47(1), 230-232 (2010-08-03)
hLDH-5 has emerged as a promising target for anti-glycolytic cancer chemotherapy. Here we report a first generation of bifunctional inhibitors, which show promising activity against hLDH-5.
Richard A Ward et al.
Journal of medicinal chemistry, 55(7), 3285-3306 (2012-03-16)
Lactate dehydrogenase A (LDHA) catalyzes the conversion of pyruvate to lactate, utilizing NADH as a cofactor. It has been identified as a potential therapeutic target in the area of cancer metabolism. In this manuscript we report our progress using fragment-based
Chrysi Xintaropoulou et al.
BMC cancer, 18(1), 636-636 (2018-06-06)
Novel therapeutic approaches are required to treat ovarian cancer and dependency on glycolysis may provide new targets for treatment. This study sought to investigate the variation of expression of molecular components (GLUT1, HKII, PKM2, LDHA) of the glycolytic pathway in
Luigi Fiume et al.
Pharmacological research, 63(4), 328-334 (2010-12-21)
Protein kinase inhibitors are a relatively new class of promising anticancer drugs, most of which exert their action by binding to the ATP site on the targeted kinases. We hypothesized that a decrease in ATP levels in neoplastic cells could
Sergi Garcia-Segura et al.
Water research, 45(9), 2975-2984 (2011-04-12)
Oxalic and oxamic acids are the ultimate and more persistent by-products of the degradation of N-aromatics by electrochemical advanced oxidation processes (EAOPs). In this paper, the kinetics and oxidative paths of these acids have been studied for several EAOPs using
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service