D57558
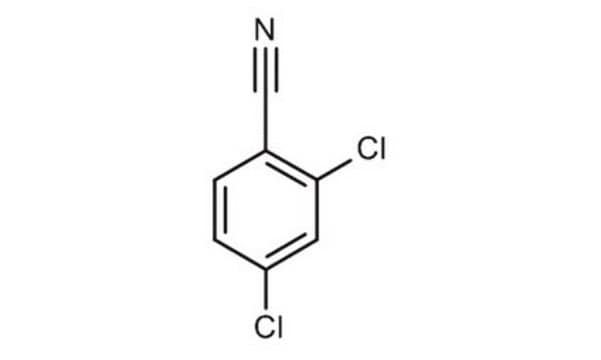
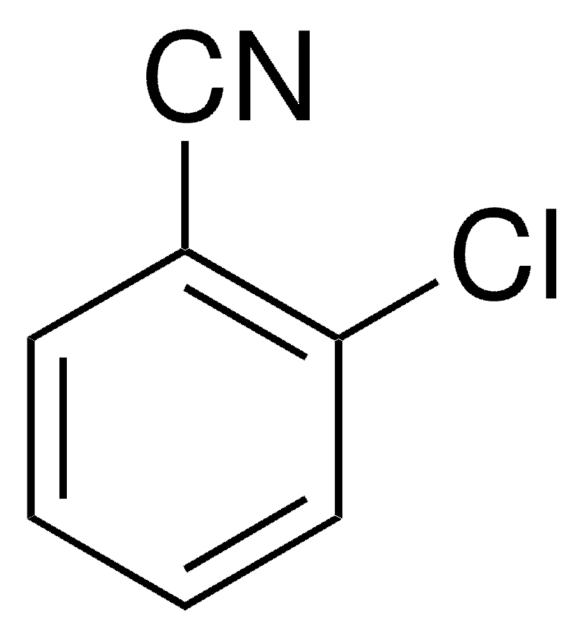
2,6-Dichlorobenzonitrile
97%
Synonym(s):
Dichlobenil
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Cl2C6H3CN
CAS Number:
Molecular Weight:
172.01
Beilstein:
1909167
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
143-146 °C (lit.)
SMILES string
Clc1cccc(Cl)c1C#N
InChI
1S/C7H3Cl2N/c8-6-2-1-3-7(9)5(6)4-10/h1-3H
InChI key
YOYAIZYFCNQIRF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Dichlorobenzonitrile can be used as a starting material to synthesize:
- 2,6-Dichlorobenzaldehyde using lithium N, N′-dimethylethylenediaminoaluminum hydride as a reducing agent.
- 5-(2,6-Dichlorophenyl)-2H-tetrazole via gold-catalyzed nucleophilic (3 + 2) cycloaddition reaction with sodium azide.
- 2,6-Dichlorobenzamide via hydrolysis using potassium tert-butoxide as a catalyst.
- Chloro-aminoindazole by reacting with hydrazine monohydrate.
- 2,6-Dichlorobenzenecarboselenoamide by treating with Woollins′ reagent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The discovery and development of a safe, practical synthesis of ABT-869
Kruger AW, et al.
Organic Process Research & Development, 13(6), 1419-1425 (2009)
Synthetic application of gold nanoparticles and auric chloride for the synthesis of 5-substituted 1 H-tetrazoles
Kumar S, et al.
Royal Society of Chemistry Advances, 5(28), 21651-21658 (2015)
Synthesis of primary arylselenoamides by reaction of aryl nitriles with Woollins' reagent
Hua Guoxiong, et al.
Organic Letters, 8(23), 5251-5254 (2006)
Transition-metal-free hydration of nitriles using potassium tert-butoxide under anhydrous conditions
Midya GC, et al.
The Journal of Organic Chemistry, 80(8), 4148-4151 (2015)
Selective conversion of aromatic nitriles to aldehydes by lithium N, N'-dimethylethylenediaminoaluminum hydride
Cha Jin-Soon, et al.
Bulletin of the Korean Chemical Society,, 23(12), 1697-1698 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service