661740
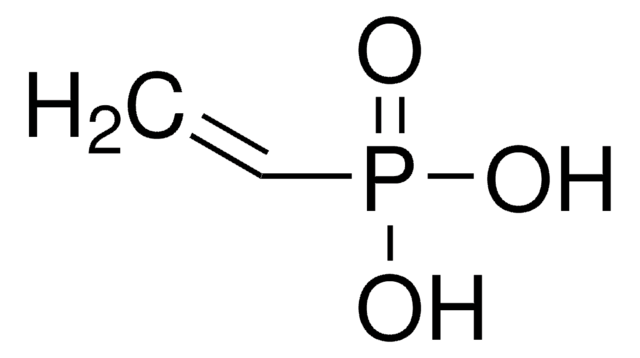
Poly(vinylphosphonic acid)
Synonym(s):
PVPA, Polyethenylphosphonic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C2H5O3P)n
CAS Number:
MDL number:
UNSPSC Code:
26111700
NACRES:
NA.23
Recommended Products
form
powder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Enabling
InChI
1S/C2H5O3P/c1-2-6(3,4)5/h2H,1H2,(H2,3,4,5)
InChI key
ZTWTYVWXUKTLCP-UHFFFAOYSA-N
Related Categories
General description
Poly(vinylphosphonic acid) (PVPA) is a polymeric diprotic acid, which can be synthesized by free-radical polymerization of VPA. It can also be synthesized by employing vinylphosphonic acid methyl ester as a monomer, followed by saponification.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Application
Poly(vinylphosphonic) acid is a versatile polyelectrolyte useful in several applications: proton conductor for fuel cells, chemical and biological sensors, biocomposite materials and surface modification and adhesion.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y E Greish et al.
Biomaterials, 22(8), 807-816 (2001-03-15)
The formation of biocompatible organic-inorganic composites by reactions between tetracalcium phosphate (Ca4(PO4)2O, TetCP) and the biomedical polymer poly(vinyl phosphonic acid) (PVPA) is described. Composites were prepared by hot pressing mixtures of these powders at 80 kpsi and 300 degrees C
Minghan Ren et al.
Analytical chemistry, 77(9), 2700-2707 (2005-04-30)
We report here a chemical sensor based on detecting the mechanical response of a thin (approximately 10-microm) polymer wire stretched across the two prongs of a wristwatch quartz tuning fork (QTF). When the fork is set to oscillate, the wire
Van Den Brand, J.; Van Gils, S.; BeenTjes, P.C.J.; Terryn, H.; Sivel, V.; de Wit, J.H.W.
Progress in Organic Coatings, 51, 339-350 (2004)
Poly (vinylphosphonic acid) and its derivatives
Macarie L and Ilia G
Progress in Polymer Science, 35(8), 1078-1092 (2010)
Sevil, F., Bozkurt A.
The Journal of Physical Chemistry, 65, 1659-1662 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service