All Photos(1)
About This Item
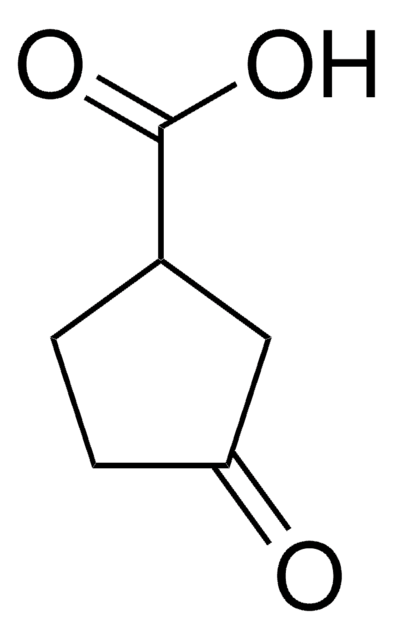
Linear Formula:
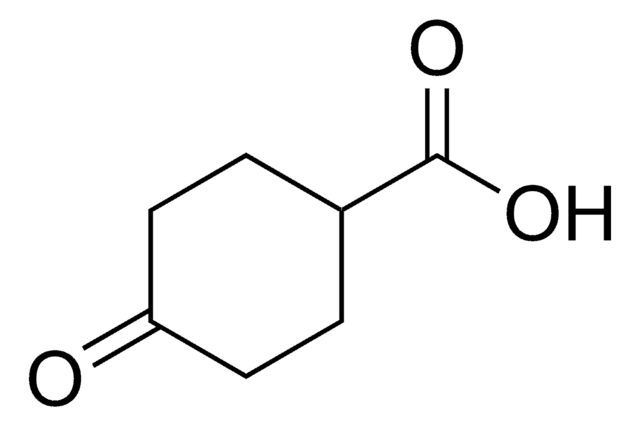
C6H9(O)CO2H
CAS Number:
Molecular Weight:
142.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
93%
SMILES string
OC(=O)C1CCCC(=O)C1
InChI
1S/C7H10O3/c8-6-3-1-2-5(4-6)7(9)10/h5H,1-4H2,(H,9,10)
InChI key
WATQNARHYZXAGY-UHFFFAOYSA-N
General description
3-Oxo-1-cyclohexanecarboxylic acid is a cyclic keto acid that can be prepared starting from 2-acetoxy-1,4-ethoxycarbonylcyclohex-1-ne.
Application
3-Oxo-1-cyclohexanecarboxylic acid (3-oxocyclohexanecarboxylic acid) may be used in the preparation of 9-(4-chlorobenzoyl)-6-methoxy-2,3,4,9-tetrahydro-1H-carbazole-2-carboxylic acid by reacting with 4-chloro-N-(4-methoxyphenyl)benzohydrazide hydrochloride via Fischer indolization.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (type 5 17?-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer.
Liedtke AJ, et al.
Journal of Medicinal Chemistry, 56(6), 2429-2446 (2013)
2,3-Donor-Acceptor-Substituted 1,3-Butadienes. Synthesis by SO2-extrusion from 3-sulfolenes and diels-alder reactions.
Hoffmann R, et al.
Advanced Synthesis & Catalysis, 336(4), 343-349 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service