All Photos(2)
About This Item
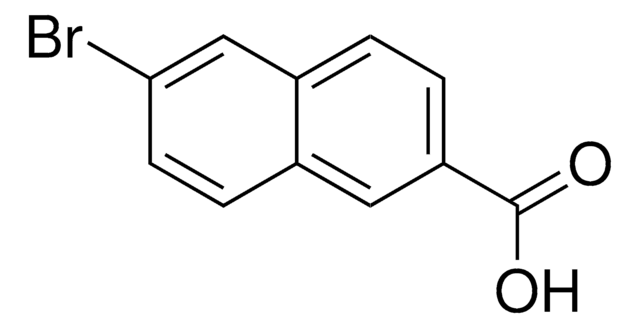
Linear Formula:
BrC10H6CO2CH3
CAS Number:
Molecular Weight:
265.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
123-126 °C (lit.)
SMILES string
COC(=O)c1ccc2cc(Br)ccc2c1
InChI
1S/C12H9BrO2/c1-15-12(14)10-3-2-9-7-11(13)5-4-8(9)6-10/h2-7H,1H3
InChI key
JEUBRLPXJZOGPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl 6-bromo-2-naphthoate undergoes aromatic Finkelstein reaction followed by hydrolysis to afford 6-iodo-2-naphthoic acid.
Application
Methyl 6-bromo-2-naphthoate may be used to synthesize:
- 6-vinyl-2-naphthalencarbaldehyde
- methyl 6-(3-tert-butyl-4-methoxyphenyl)-2-naphthoate
- methyl 6-[3-tert-butyl-4-[(tert-butyldiethylsilyl)oxy]-phenyl]-2-naphthoate
- methyl 6-[3-(1-adamantyl)-4-[(tert-butyldimethylsilyl)-oxy]phenyl]-2-naphthoate
- methyl 6-[3-(1-adamantyl)-4-[[(2,3-dimethyl-1,3-dioxolan-4-yl)methylloxy]phenyl]-2-naphthoate
- 2-bromo-6-(bromomethyl)naphthalene
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Phil M Pithan et al.
Beilstein journal of organic chemistry, 12, 854-862 (2016-06-25)
Cationic biaryl derivatives were synthesized by Suzuki-Miyaura coupling of 3-bromonaphtho[1,2-b]quinolizinium bromide with arylboronic acids. The resulting cationic biaryl derivatives exhibit pronounced fluorosolvatochromic properties. First photophysical studies in different solvents showed that the emission energy of the biaryl derivatives decreases with
Mark W Irvine et al.
Journal of medicinal chemistry, 55(1), 327-341 (2011-11-25)
Competitive N-methyl-d-aspartate receptor (NMDAR) antagonists bind to the GluN2 subunit, of which there are four types (GluN2A-D). We report that some N(1)-substituted derivatives of cis-piperazine-2,3-dicarboxylic acid display improved relative affinity for GluN2C and GluN2D versus GluN2A and GluN2B. These derivatives
Carboxy-1, 4-phenylenevinylene-and carboxy-2, 6-naphthylene-vinylene unsymmetrical substituted zinc phthalocyanines for dye-sensitized solar cells.
Silvestri F, et al.
Journal of Porphyrins and Phthalocyanines, 13(03), 369-375 (2009)
B Charpentier et al.
Journal of medicinal chemistry, 38(26), 4993-5006 (1995-12-22)
The retinoic acid receptors (RARs) transduce retinoid dependant gene regulation, and many biological effects of retinoids are mediated through binding and activation of three closely related receptor subtypes (RAR alpha, RAR beta, and RAR gamma). In order to investigate the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



